Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis
- PMID: 29414687
- PMCID: PMC5807007
- DOI: 10.1016/j.cmet.2018.01.001
Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis
Abstract
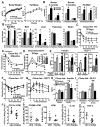
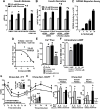
Palmitic acid hydroxystearic acids (PAHSAs) are endogenous lipids with anti-diabetic and anti-inflammatory effects. PAHSA levels are reduced in serum and adipose tissue of insulin-resistant people and high-fat diet (HFD)-fed mice. Here, we investigated whether chronic PAHSA treatment enhances insulin sensitivity and which receptors mediate PAHSA effects. Chronic PAHSA administration in chow- and HFD-fed mice raises serum and tissue PAHSA levels ∼1.4- to 3-fold. This improves insulin sensitivity and glucose tolerance without altering body weight. PAHSA administration in chow-fed, but not HFD-fed, mice augments insulin and glucagon-like peptide (GLP-1) secretion. PAHSAs are selective agonists for GPR40, increasing Ca+2 flux, but not intracellular cyclic AMP. Blocking GPR40 reverses improvements in glucose tolerance and insulin sensitivity in PAHSA-treated chow- and HFD-fed mice and directly inhibits PAHSA augmentation of glucose-stimulated insulin secretion in human islets. In contrast, GLP-1 receptor blockade in PAHSA-treated chow-fed mice reduces PAHSA effects on glucose tolerance, but not on insulin sensitivity. Thus, PAHSAs activate GPR40, which is involved in their beneficial metabolic effects.
Keywords: FAHFA; GLP-1 receptor; GPR40; glucose-insulin homeostasis; human islets; insulin secretion; palmitic acid hydroxystearic acid; type 2 diabetes.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
“The authors declare no competing interests.”
Figures


References
-
- Aroda VR, Henry R, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ. Efficacy of GLP-1R agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247– 1258. - PubMed
-
- Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in T2D mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. - PubMed
-
- Campbell JE, Drucker DJ. Pharmacology physiology and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

