Glycation of human serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide
- PMID: 29414771
- PMCID: PMC5880132
- DOI: 10.1074/jbc.M117.815274
Glycation of human serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide
Abstract
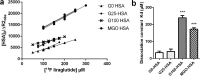
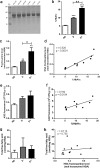
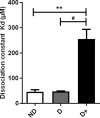
The long-acting glucagon-like peptide-1 analogue liraglutide has proven efficiency in the management of type 2 diabetes and also has beneficial effects on cardiovascular diseases. Liraglutide's protracted action highly depends on its capacity to bind to albumin via its palmitic acid part. However, in diabetes, albumin can undergo glycation, resulting in impaired drug binding. Our objective in this study was to assess the impact of human serum albumin (HSA) glycation on liraglutide affinity. Using fluorine labeling of the drug and 19F NMR, we determined HSA affinity for liraglutide in two glycated albumin models. We either glycated HSA in vitro by incubation with glucose (G25- or G100-HSA) or methylglyoxal (MGO-HSA) or purified in vivo glycated HSA from the plasma of diabetic patients with poor glycemic control. Nonglycated commercial HSA (G0-HSA) and HSA purified from plasma of healthy individuals served as controls. We found that glycation decreases affinity for liraglutide by 7-fold for G100-HSA and by 5-fold for MGO-HSA compared with G0-HSA. A similarly reduced affinity was observed for HSA purified from diabetic individuals compared with HSA from healthy individuals. Our results reveal that glycation significantly impairs HSA affinity to liraglutide and confirm that glycation contributes to liraglutide's variable therapeutic efficiency, depending on diabetes stage. Because diabetes is a progressive disease, the effect of glycated albumin on liraglutide affinity found here is important to consider when diabetes is managed with this drug.
Keywords: albumin; diabetes; glycation; liraglutide; nuclear magnetic resonance (NMR); protein drug interaction; spectroscopy.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- International Diabetes Federation (2015) International Diabetes Federation diabetes atlas, 5th Ed., International Diabetes Federation, Brussels, Belgium
-
- Steensgaard D. B., Thomsen J. K., Olsen H. B., and Knudsen L. B. (2008) The molecular basis for the delayed absorption of the once-daily human GLP-1 analogue, liraglutide. Diabetes 57, A164
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

