Polymicrobial sepsis and non-specific immunization induce adaptive immunosuppression to a similar degree
- PMID: 29415028
- PMCID: PMC5802895
- DOI: 10.1371/journal.pone.0192197
Polymicrobial sepsis and non-specific immunization induce adaptive immunosuppression to a similar degree
Abstract
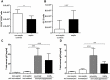
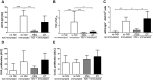
Sepsis is frequently complicated by a state of profound immunosuppression, in its extreme form known as immunoparalysis. We have studied the role of the adaptive immune system in the murine acute peritonitis model. To read out adaptive immunosuppression, we primed post-septic and control animals by immunization with the model antigen TNP-ovalbumin in alum, and measured the specific antibody-responses via ELISA and ELISpot assay as well as T-cell responses in a proliferation assay after restimulation. Specific antibody titers, antibody affinity and plasma cell counts in the bone marrow were reduced in post-septic animals. The antigen-induced splenic proliferation was also impaired. The adaptive immunosuppression was positively correlated with an overwhelming general antibody response to the septic insult. Remarkably, antigen "overload" by non-specific immunization induced a similar degree of adaptive immunosuppression in the absence of sepsis. In both settings, depletion of regulatory T cells before priming reversed some parameters of the immunosuppression. In conclusion, our data show that adaptive immunosuppression occurs independent of profound systemic inflammation and life-threatening illness.
Conflict of interest statement
Figures


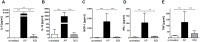

References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29(7):1303–10. . - PubMed
-
- Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101(1):36–47. . - PubMed
-
- Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29(4):617 doi: 10.1016/j.ccm.2008.06.010 . - DOI - PMC - PubMed
-
- Cavassani KA, Carson WFt, Moreira AP, Wen H, Schaller MA, Ishii M, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115(22):4403–11. doi: 10.1182/blood-2009-09-241083 . - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

