Mechanical and phytochemical protection mechanisms of Calligonum comosum in arid deserts
- PMID: 29415032
- PMCID: PMC5802934
- DOI: 10.1371/journal.pone.0192576
Mechanical and phytochemical protection mechanisms of Calligonum comosum in arid deserts
Abstract
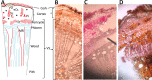
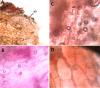
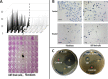
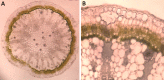
Unlike animals, plants are sessile organisms, lacking circulating antibodies and specialized immune cells and are exposed to various harsh environmental conditions that make them at risk of being attacked by different pathogens and herbivores. Plants produce chemo-signals to respond to the surroundings and be able to distinguish between harmless and harmful signals. In this study, the production of phytochemicals as plant signaling mechanisms and their defensive roles in disease resistance and repelling herbivores are examined in Calligonum comosum. C. comosum is a leafless standalone perennial shrub widespread in sand dunes. The plant has the ability to survive the drastic environmental conditions of the arid/ hyperarid deserts of the Arabia. Structural anatomy and phytochemicals analyses were used to identify both mechanical and chemical defensive mechanisms in C. comosum. Microscopy-based investigations indicated that stems of this species developed hard structures in its outer layers including sclerenchyma and cluster crystals of calcium oxalate (CaOx). Sclerenchyma and CaOx are difficult to be eaten by herbivores and insects and can harm their mouthparts. On the other hand, the plant developed both short-distance (local) and long-distance (systematic over limited sphere) phytochemicals-producing cells located at its outer regions that is surrounding the inner nutrient-rich vascular system (VS). Local chemical was represented by phenolic idioblasts that were released in response to plant cutting. Systematic chemical was represented by toxic volatile oil containing ~50% benzaldehyde derivative (cuminaldehyde). The oil caused strong killing effect on both mammalian cells and microbial pathogens via either direct addition or indirect exposure to its vapor. The plants lost the oil content and allowed fungal growth once cut and dried. The localization of both defensive mechanisms to the outer region of the plant seemed to protect the inner nutrient-rich VS and hence maintained the plant survival. Surprisingly, in relation to traditional folklore use as medicine, local people use only green parts of the plant and only during the winter, where the plant found devoid of volatile oil and phenolic idioblasts. Moreover, it turns into recommendations for local people to avoid any health problems caused by the plant supply.
Conflict of interest statement
Figures






References
-
- Mithöfer A, Boland W, Maffei ME. Chemical Ecology of Plant–Insect Interactions Annual plant reviews volume 34: Molecular aspects of plant disease resistance: Wiley-Blackwell; 2009. p. 261–91.
-
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, et al. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012;7(10):1306–20. doi: 10.4161/psb.21663 PubMed PMID: PMC3493419. - DOI - PMC - PubMed
-
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323 doi: 10.1038/nature05286 - DOI - PubMed
-
- Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms. New Phytol. 1994;127(4):617–33. doi: 10.1111/j.1469-8137.1994.tb02968.x - DOI - PubMed
-
- Karban R, English-Loeb G, Hougen-Eitzman D. Mite vaccinations for sustainable management of spider mites in vineyards. Ecol Appl. 1997;7:183–93.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

