Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans
- PMID: 29415062
- PMCID: PMC5802856
- DOI: 10.1371/journal.pone.0191360
Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans
Abstract
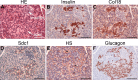
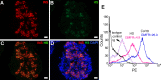
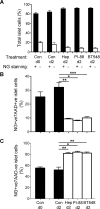
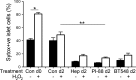
Type 1 diabetes (T1D) is an autoimmune disease in which insulin-producing beta cells in pancreatic islets are progressively destroyed. Clinical trials of immunotherapies in recently diagnosed T1D patients have only transiently and partially impacted the disease course, suggesting that other approaches are required. Our previous studies have demonstrated that heparan sulfate (HS), a glycosaminoglycan conventionally expressed in extracellular matrix, is present at high levels inside normal mouse beta cells. Intracellular HS was shown to be critical for beta cell survival and protection from oxidative damage. T1D development in Non-Obese Diabetic (NOD) mice correlated with loss of islet HS and was prevented by inhibiting HS degradation by the endoglycosidase, heparanase. In this study we investigated the distribution of HS and heparan sulfate proteoglycan (HSPG) core proteins in normal human islets, a role for HS in human beta cell viability and the clinical relevance of intra-islet HS and HSPG levels, compared to insulin, in human T1D. In normal human islets, HS (identified by 10E4 mAb) co-localized with insulin but not glucagon and correlated with the HSPG core proteins for collagen type XVIII (Col18) and syndecan-1 (Sdc1). Insulin-positive islets of T1D pancreases showed significant loss of HS, Col18 and Sdc1 and heparanase was strongly expressed by islet-infiltrating leukocytes. Human beta cells cultured with HS mimetics showed significantly improved survival and protection against hydrogen peroxide-induced death, suggesting that loss of HS could contribute to beta cell death in T1D. We conclude that HS depletion in beta cells, possibly due to heparanase produced by insulitis leukocytes, may function as an important mechanism in the pathogenesis of human T1D. Our findings raise the possibility that intervention therapy with dual activity HS replacers/heparanase inhibitors could help to protect the residual beta cell mass in patients recently diagnosed with T1D.
Conflict of interest statement
Figures



Similar articles
-
Islet heparan sulfate but not heparan sulfate proteoglycan core protein is lost during islet isolation and undergoes recovery post-islet transplantation.Am J Transplant. 2015 Nov;15(11):2851-64. doi: 10.1111/ajt.13366. Epub 2015 Jun 23. Am J Transplant. 2015. PMID: 26104150
-
Heparanase and Type 1 Diabetes.Adv Exp Med Biol. 2020;1221:607-630. doi: 10.1007/978-3-030-34521-1_24. Adv Exp Med Biol. 2020. PMID: 32274728 Review.
-
Unexpected new roles for heparanase in Type 1 diabetes and immune gene regulation.Matrix Biol. 2013 Jun 24;32(5):228-33. doi: 10.1016/j.matbio.2013.02.007. Epub 2013 Mar 13. Matrix Biol. 2013. PMID: 23499527 Review.
-
Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes.J Clin Invest. 2012 Jan;122(1):132-41. doi: 10.1172/JCI46177. Epub 2011 Dec 19. J Clin Invest. 2012. PMID: 22182841 Free PMC article.
-
Heparan sulfate proteoglycans in beta cells provide a critical link between endoplasmic reticulum stress, oxidative stress and type 2 diabetes.PLoS One. 2021 Jun 4;16(6):e0252607. doi: 10.1371/journal.pone.0252607. eCollection 2021. PLoS One. 2021. PMID: 34086738 Free PMC article.
Cited by
-
Loss of Hs3st3a1 or Hs3st3b1 enzymes alters heparan sulfate to reduce epithelial morphogenesis and adult salivary gland function.Matrix Biol. 2021 Sep;103-104:37-57. doi: 10.1016/j.matbio.2021.10.002. Epub 2021 Oct 12. Matrix Biol. 2021. PMID: 34653670 Free PMC article.
-
Inhibition of heparanase protects against pancreatic beta cell death in streptozotocin-induced diabetic mice via reducing intra-islet inflammatory cell infiltration.Br J Pharmacol. 2020 Oct;177(19):4433-4447. doi: 10.1111/bph.15183. Epub 2020 Aug 19. Br J Pharmacol. 2020. PMID: 32608014 Free PMC article.
-
SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment.Cell Metab. 2021 Aug 3;33(8):1565-1576.e5. doi: 10.1016/j.cmet.2021.05.013. Epub 2021 May 18. Cell Metab. 2021. PMID: 34081912 Free PMC article.
-
Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications.Theranostics. 2022 Jan 1;12(3):1342-1372. doi: 10.7150/thno.65778. eCollection 2022. Theranostics. 2022. PMID: 35154494 Free PMC article. Review.
-
Pancreas Pathology During the Natural History of Type 1 Diabetes.Curr Diab Rep. 2018 Oct 6;18(11):124. doi: 10.1007/s11892-018-1084-3. Curr Diab Rep. 2018. PMID: 30293191 Review.
References
-
- Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes-considerations for attempts to prevent and reverse the disease. Diabetes Care. 2015; 38(6): 979–988. doi: 10.2337/dc15-0144 . - DOI - PMC - PubMed
-
- Battaglia M, Atkinson MA. The streetlight effect in type 1 diabetes. Diabetes. 2015; 64(4): 1081–1090. doi: 10.2337/db14-1208 . - DOI - PMC - PubMed
-
- In't Veld P. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Seminars in Immunopathology. 2014; 36(5): 569–579. doi: 10.1007/s00281-014-0438-4 . - DOI - PMC - PubMed
-
- Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013; 56(11): 2541–2543. doi: 10.1007/s00125-013-3043-5 . - DOI - PubMed
-
- In't Veld P. Insulitis in human type 1 diabetes: The quest for an elusive lesion. Islets. 2011; 3(4): 131–138. doi: 10.4161/isl.3.4.15728 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

