MR-guided transcranial focused ultrasound safely enhances interstitial dispersion of large polymeric nanoparticles in the living brain
- PMID: 29415084
- PMCID: PMC5802894
- DOI: 10.1371/journal.pone.0192240
MR-guided transcranial focused ultrasound safely enhances interstitial dispersion of large polymeric nanoparticles in the living brain
Abstract
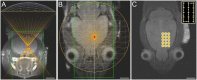
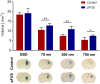
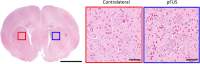
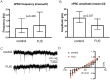
Generating spatially controlled, non-destructive changes in the interstitial spaces of the brain has a host of potential clinical applications, including enhancing the delivery of therapeutics, modulating biological features within the tissue microenvironment, altering fluid and pressure dynamics, and increasing the clearance of toxins, such as plaques found in Alzheimer's disease. Recently we demonstrated that ultrasound can non-destructively enlarge the interstitial spaces of the brain ex vivo. The goal of the current study was to determine whether these effects could be reproduced in the living brain using non-invasive, transcranial MRI-guided focused ultrasound (MRgFUS). The left striatum of healthy rats was treated using MRgFUS. Computer simulations facilitated treatment planning, and targeting was validated using MRI acoustic radiation force impulse imaging. Following MRgFUS treatments, Evans blue dye or nanoparticle probes were infused to assess changes in the interstitial space. In MRgFUS-treated animals, enhanced dispersion was observed compared to controls for 70 nm (12.8 ± 0.9 mm3 vs. 10.6 ± 1.0 mm3, p = 0.01), 200 nm (10.9 ± 1.4 mm3 vs. 7.4 ± 0.7 mm3, p = 0.01) and 700 nm (7.5 ± 0.4 mm3 vs. 5.4 ± 1.2 mm3, p = 0.02) nanoparticles, indicating enlargement of the interstitial spaces. No evidence of significant histological or electrophysiological injury was identified. These findings suggest that transcranial ultrasound can safely and effectively modulate the brain interstitium and increase the dispersion of large therapeutic entities such as particulate drug carriers or modified viruses. This has the potential to expand the therapeutic uses of MRgFUS.
Conflict of interest statement
Figures



References
-
- Wolak DJ, Thorne RG. Diffusion of macromolecules in the brain: implications for drug delivery. Mol Pharm. 2013;10(5):1492–504. doi: 10.1021/mp300495e . - DOI - PMC - PubMed
-
- Thorne RG, Hrabetova S, Nicholson C. Diffusion of epidermal growth factor in rat brain extracellular space measured by integrative optical imaging. J Neurophysiol. 2004;92(6):3471–81. Epub 2004/07/23. doi: 10.1152/jn.00352.2004 . - DOI - PubMed
-
- Vargova L, Sykova E. Extracellular space diffusion and extrasynaptic transmission. Physiol Res. 2008;57 Suppl 3:S89–99. Epub 2008/05/17. . - PubMed
-
- Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, et al. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309(5733):446–51. doi: 10.1126/science.1108239 . - DOI - PMC - PubMed
-
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25(33):7538–47. Epub 2005/08/19. doi: 10.1523/JNEUROSCI.1927-05.2005 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

