Metabolic Effects of Preexposure Prophylaxis With Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine
- PMID: 29415175
- PMCID: PMC6051460
- DOI: 10.1093/cid/ciy083
Metabolic Effects of Preexposure Prophylaxis With Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine
Abstract
Background: Antiretroviral drugs have been associated with changes in lipids, fat mass and dat distribution. Tenofovir disoproxil fumarate (TDF) has been shown to have a more favorable metabolic profile than other drugs in its class. However, the metabolic effects of TDF in preexposure prophylaxis (PrEP) are unknown.
Methods: We evaluated the effects of TDF/emtricitabine (FTC) on lipids and body composition in a blinded, placebo-controlled PrEP trial. Participants enrolled in a metabolic subcohort (N = 251, TDF/FTC; N = 247, placebo) consented to fasting lipid panels, dual-energy X-ray absorptiometry scans for body composition, and pharmacologic testing of drug metabolites at baseline and every 24 weeks thereafter.
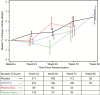
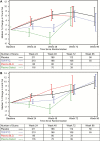
Results: Lean body mass was stable and unaffected by TDF/FTC. Body weight increased in both groups but was lower on TDF/FTC through week 72. This difference was explained by lower fat accumulation on TDF/FTC. The net median percent difference (standard error, P value) for TDF/FTC vs placebo at week 24 was -0.8% (0.4%, P = .02), +0.3% (0.4%, P = .46), and -3.8% (1.4%, P = .009) for total, lean, and fat mass, respectively. There was no apparent differential regional fat accumulation on TDF/FTC. Decreases in cholesterol, but not triglycerides, were seen in TDF/FTC participants, with detectable drug levels compared to placebo.
Conclusions: TDF/FTC for PrEP showed cholesterol reductions and appeared to transiently suppress the accumulation of weight and body fat compared to placebo. There was no evidence of altered fat distribution or lipodystrophy during daily oral TDF/FTC PrEP.
Clinical trials registration: NCT00458393.
Figures


References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. ; TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, et al. ; Bangkok Tenofovir Study Group Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. - PubMed
-
- Molina JM, Capitant C, Spire B, et al. ; ANRS IPERGAY Study Group On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

