HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease
- PMID: 29415205
- PMCID: PMC5837793
- DOI: 10.1093/brain/awx375
HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease
Abstract
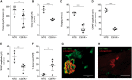
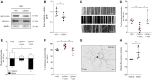
Peripheral nerve axons require a well-organized axonal microtubule network for efficient transport to ensure the constant crosstalk between soma and synapse. Mutations in more than 80 different genes cause Charcot-Marie-Tooth disease, which is the most common inherited disorder affecting peripheral nerves. This genetic heterogeneity has hampered the development of therapeutics for Charcot-Marie-Tooth disease. The aim of this study was to explore whether histone deacetylase 6 (HDAC6) can serve as a therapeutic target focusing on the mutant glycyl-tRNA synthetase (GlyRS/GARS)-induced peripheral neuropathy. Peripheral nerves and dorsal root ganglia from the C201R mutant Gars mouse model showed reduced acetylated α-tubulin levels. In primary dorsal root ganglion neurons, mutant GlyRS affected neurite length and disrupted normal mitochondrial transport. We demonstrated that GlyRS co-immunoprecipitated with HDAC6 and that this interaction was blocked by tubastatin A, a selective inhibitor of the deacetylating function of HDAC6. Moreover, HDAC6 inhibition restored mitochondrial axonal transport in mutant GlyRS-expressing neurons. Systemic delivery of a specific HDAC6 inhibitor increased α-tubulin acetylation in peripheral nerves and partially restored nerve conduction and motor behaviour in mutant Gars mice. Our study demonstrates that α-tubulin deacetylation and disrupted axonal transport may represent a common pathogenic mechanism underlying Charcot-Marie-Tooth disease and it broadens the therapeutic potential of selective HDAC6 inhibition to other genetic forms of axonal Charcot-Marie-Tooth disease.
Figures





References
-
- Achilli F, Bros-Facer V, Williams HP, Banks GT, AlQatari M, Chia R, et al. An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis Model Mech 2009; 2: 359–73. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

