Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond
- PMID: 29415512
- PMCID: PMC5836077
- DOI: 10.3390/cancers10020045
Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond
Abstract
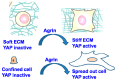
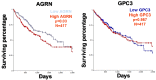
In addition to the structural and scaffolding role, the extracellular matrix (ECM) is emerging as a hub for biomechanical signal transduction that is frequently relayed to intracellular sensors to regulate diverse cellular processes. At a macroscopic scale, matrix rigidity confers long-ranging effects contributing towards tissue fibrosis and cancer. The transcriptional co-activators YAP/TAZ, better known as the converging effectors of the Hippo pathway, are widely recognized for their new role as nuclear mechanosensors during organ homeostasis and cancer. Still, how YAP/TAZ senses these "stiffness cues" from the ECM remains enigmatic. Here, we highlight the recent perspectives on the role of agrin in mechanosignaling from the ECM via antagonizing the Hippo pathway to activate YAP/TAZ in the contexts of cancer, neuromuscular junctions, and cardiac regeneration.
Keywords: Hippo pathway; YAP/TAZ; agrin; cardiac regeneration; extracellular matrix; liver cancer; mechanotransduction; neuromuscular junctions.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures




Similar articles
-
Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway.Cell Rep. 2017 Mar 7;18(10):2464-2479. doi: 10.1016/j.celrep.2017.02.041. Cell Rep. 2017. PMID: 28273460
-
YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer.Int J Mol Sci. 2018 Nov 20;19(11):3674. doi: 10.3390/ijms19113674. Int J Mol Sci. 2018. PMID: 30463366 Free PMC article. Review.
-
An Agrin-YAP/TAZ Rigidity Sensing Module Drives EGFR-Addicted Lung Tumorigenesis.Adv Sci (Weinh). 2025 May;12(20):e2413443. doi: 10.1002/advs.202413443. Epub 2025 Mar 31. Adv Sci (Weinh). 2025. PMID: 40165020 Free PMC article.
-
Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression.Front Cell Dev Biol. 2021 May 24;9:673599. doi: 10.3389/fcell.2021.673599. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34109179 Free PMC article. Review.
-
The biology of YAP/TAZ: hippo signaling and beyond.Physiol Rev. 2014 Oct;94(4):1287-312. doi: 10.1152/physrev.00005.2014. Physiol Rev. 2014. PMID: 25287865 Review.
Cited by
-
Rigid Tissue Increases Cytoplasmic pYAP Expression in Pre-Malignant Stage of Lung Squamous Cell Carcinoma (SCC) In Vivo.Curr Issues Mol Biol. 2022 Sep 29;44(10):4528-4539. doi: 10.3390/cimb44100310. Curr Issues Mol Biol. 2022. PMID: 36286025 Free PMC article.
-
Multiple MuSK signaling pathways and the aging neuromuscular junction.Neurosci Lett. 2020 Jul 13;731:135014. doi: 10.1016/j.neulet.2020.135014. Epub 2020 Apr 28. Neurosci Lett. 2020. PMID: 32353380 Free PMC article. Review.
-
Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression.Gut. 2022 Jan 10;71(10):2043-68. doi: 10.1136/gutjnl-2021-324994. Online ahead of print. Gut. 2022. PMID: 35012996 Free PMC article.
-
Agrin expression is correlated with tumor development and poor prognosis in cholangiocarcinoma.J Int Med Res. 2021 May;49(5):3000605211009722. doi: 10.1177/03000605211009722. J Int Med Res. 2021. PMID: 34018826 Free PMC article.
-
Agrin has a pathological role in the progression of oral cancer.Br J Cancer. 2018 Jun;118(12):1628-1638. doi: 10.1038/s41416-018-0135-5. Epub 2018 Jun 6. Br J Cancer. 2018. PMID: 29872149 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

