Mobile critical care recovery program (m-CCRP) for acute respiratory failure survivors: study protocol for a randomized controlled trial
- PMID: 29415760
- PMCID: PMC5803999
- DOI: 10.1186/s13063-018-2449-2
Mobile critical care recovery program (m-CCRP) for acute respiratory failure survivors: study protocol for a randomized controlled trial
Abstract
Background: Patients admitted to intensive care units (ICU) with acute respiratory failure (ARF) face chronic complications that can impede return to normal daily function. A mobile, collaborative critical care model may enhance the recovery of ARF survivors.
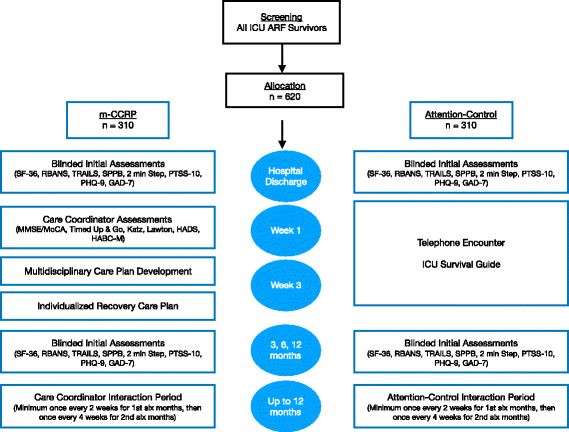
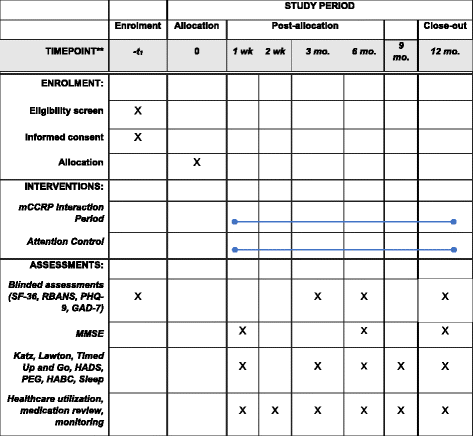
Methods: The Mobile Critical Care Recovery Program (m-CCRP) study is a two arm, randomized clinical trial. We will randomize 620 patients admitted to the ICU with acute respiratory failure requiring mechanical ventilation in a 1:1 ratio to one of two arms (310 patients per arm) - m-CCRP intervention versus attention control. Those in the intervention group will meet with a care coordinator after hospital discharge in predetermined intervals to aid in the recovery process. Baseline assessments and personalized goal setting will be used to develop an individualized care plan for each patient after discussion with an interdisciplinary team. The attention control arm will receive printed material and telephone reminders emphasizing mobility and management of chronic conditions. Duration of the intervention and follow-up is 12 months post-randomization. Our primary aim is to assess the efficacy of m-CCRP in improving the quality of life of ARF survivors at 12 months. Secondary aims of the study are to evaluate the efficacy of m-CCRP in improving function (cognitive, physical, and psychological) of ARF survivors and to determine the efficacy of m-CCRP in reducing acute healthcare utilization.
Discussion: The proposed randomized controlled trial will evaluate the efficacy of a collaborative critical care recovery program in accomplishing the Institute of Healthcare Improvement's triple aims of better health, better care, at lower cost. We have developed a collaborative critical care model to promote ARF survivors' recovery from the physical, psychological, and cognitive impacts of critical illness. In contrast to a single disease focus and clinic-based access, m-CCRP represents a comprehensive, accessible, mobile, ahead of the curve intervention, focused on the multiple aspects of the unique recovery needs of ARF survivors.
Trial registration: NCT03053245 , clinicaltrials.gov, registered February 1, 2017.
Keywords: Cognitive impairment; Cognitive training; Critical care; Delirium; ICU survivorship; Physical activity; Post-intensive care syndrome; Quality of life.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
The m-CCRP study received ethical approval from Indiana University’s Institutional Review Board (IRB #160595004), covering all participating sites. Important changes to the protocol will be submitted to the IRB for review. Informed consent will be obtained from all participants in the trial.
Consent for publication
Consent forms for the trial include consent for publication of results in peer-reviewed journals.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Esperson K, Antonsen K, Jensen H. Capacity problems in Danish Intensive Care Units. Ugeskr Laeger. 2007;169:710–2. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

