Guidance for Pediatric Familial Hypercholesterolemia 2017
- PMID: 29415907
- PMCID: PMC6005224
- DOI: 10.5551/jat.CR002
Guidance for Pediatric Familial Hypercholesterolemia 2017
Abstract
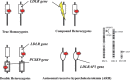
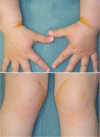
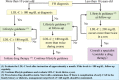
This paper describes consensus statement by Joint Working Group by Japan Pediatric Society and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial Hypercholesterolemia (FH) in order to improve prognosis of FH.FH is a common genetic disease caused by mutations in genes related to low density lipoprotein (LDL) receptor pathway. Because patients with FH have high LDL cholesterol (LDL-C) levels from the birth, atherosclerosis begins and develops during childhood which determines the prognosis. Therefore, in order to reduce their lifetime risk for cardiovascular disease, patients with FH need to be diagnosed as early as possible and appropriate treatment should be started.Diagnosis of pediatric heterozygous FH patients is made by LDL-C ≥140 mg/dL, and family history of FH or premature CAD. When the diagnosis is made, they need to improve their lifestyle including diet and exercise which sometimes are not enough to reduce LDL-C levels. For pediatric FH aged ≥10 years, pharmacotherapy needs to be considered if the LDL-C level is persistently above 180 mg/dL. Statins are the first line drugs starting from the lowest dose and are increased if necessary. The target LDL-C level should ideally be <140 mg/dL. Assessment of atherosclerosis is mainly performed by noninvasive methods such as ultrasound.For homozygous FH patients, the diagnosis is made by existence of skin xanthomas or tendon xanthomas from infancy, and untreated LDL-C levels are approximately twice those of heterozygous FH parents. The responsiveness to pharmacotherapy should be ascertained promptly and if the effect of treatment is not enough, LDL apheresis needs to be immediately initiated.
Keywords: Diagnostic criteria; Guidance; Heterozygote; Homozygote; LDL apheresis; Lifestyle; Pediatric familial hypercholesterolemia; Pharmacological therapy.
Conflict of interest statement
Employment/Leadership position/Advisory role: Akira Ohtake; Kowa Pharmaceutical Co. Ltd. Honoraria: Mariko Harada-Shiba; Kowa Pharmaceutical Co. Ltd, Amgen Astellas BioPharma K.K., Sanofi K.K. Akira Ohtake; Novo Nordisk Pharma Ltd., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., JCR Pharmaceuticals Co., Ltd., Pfizer Japan Inc., Meiji Seika Pharma Co., Ltd., Sanofi K.K.. Masatsune Ogura; Astellas Pharma Inc., Amgen Astellas BioPharma K.K., Sanofi K.K.. Shizuya Yamashita; Kowa Company, Ltd., MSD K.K./Merck & Co, Bayer Yakuhin, Ltd, Skylight Biotech, Inc., Medical Review Co., Ltd., Amgen Astellas Bio-Pharma K.K., Pfizer Japan Inc., Dai Nippon Printing Co., Ltd.. Atsushi Nohara; Astellas Pharma Inc., Amgen Astellas BioPharma K.K., Sanofi K.K.. Fees for promotional materials: Shizuya Yamashita; Kowa Company, Ltd., Bayer Yakuhin, Ltd, Sanwa Kagaku Kenkyusho Co.,Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Medex Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd.. Research funding: Mariko Harada-Shiba; Astellas Pharma Inc., Kaneka Medix Corporation. Atsushi Nohara; Shionogi & Co., Ltd., Aegerion Pharmaceuticals, Inc., Alexion. Shizuya Yamashita; Tak eda Pharmaceutical Company Limited., Astellas Pharma Inc., Mochida Pharmaceutical Co., Ltd.. Others: Shizuya Yamashita; Department of Community Medicine & Department of Cardiovascular Medicine Osaka University Graduate School of Medicine, Rinku General Medical Center, Kaizuka City Hospital
This is an English version of the Guidance for pediatric familial hypercholesterolemia 2017 in Japan published in Japanese in January, 2017.
Figures



References
-
- Goldstein JL, Hobbs HH, Brown MS: Familial hypercholesterolemia, 8th edn, vol. 2 McGraw-Hill: New York, 2001; 2863-2913
-
- Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J, Hokuriku FH Study Group : Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 - PubMed
-
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS: Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA, 2003; 290: 2271-2276 - PubMed
-
- Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group: Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Arterioscler Thromb, 1993; 13: 1291-1298 - PubMed
-
- Wiegman A, Hutten BA, de Groot E, Rosenberg J, Bakker HD, Buller HR, Sijbrands EJ, Kastelein JJ: Efficacy and safety of statin therapy in children with familial hypercholesterolemia. JAMA, 2004; 292: 331-337 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

