PilVax - a novel peptide delivery platform for the development of mucosal vaccines
- PMID: 29416095
- PMCID: PMC5803258
- DOI: 10.1038/s41598-018-20863-7
PilVax - a novel peptide delivery platform for the development of mucosal vaccines
Abstract
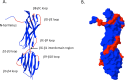
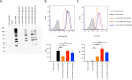
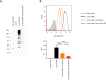
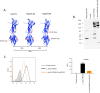
Peptide vaccines are an attractive strategy to engineer the induction of highly targeted immune responses and avoid potentially allergenic and/or reactogenic protein regions. However, peptides by themselves are often unstable and poorly immunogenic, necessitating the need for an adjuvant and a specialised delivery system. We have developed a novel peptide delivery platform (PilVax) that allows the presentation of a stabilised and highly amplified peptide as part of the group A streptococcus serotype M1 pilus structure (PilM1) on the surface of the non-pathogenic bacterium Lactococcus lactis. To show proof of concept, we have successfully inserted the model peptide Ova324-339 into 3 different loop regions of the backbone protein Spy0128, which resulted in the assembly of the pilus containing large numbers of peptide on the surface of L. lactis. Intranasal immunisation of mice with L. lactis PilM1-Ova generated measurable Ova-specific systemic and mucosal responses (IgA and IgG). Furthermore, we show that multiple peptides can be inserted into the PilVax platform and that peptides can also be incorporated into structurally similar, but antigenically different pilus structures. PilVax may be useful as a cost-effective platform for the development of peptide vaccines against a variety of important human pathogens.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
PilVax: A Novel Platform for the Development of Mucosal Vaccines.Methods Mol Biol. 2022;2412:399-410. doi: 10.1007/978-1-0716-1892-9_20. Methods Mol Biol. 2022. PMID: 34918257
-
PilVax, a novel Lactococcus lactis-based mucosal vaccine platform, stimulates systemic and mucosal immune responses to Staphylococcus aureus.Immunol Cell Biol. 2020 May;98(5):369-381. doi: 10.1111/imcb.12325. Epub 2020 Apr 9. Immunol Cell Biol. 2020. PMID: 32150301
-
Intranasal immunization with Ag85B peptide 25 displayed on Lactococcus lactis using the PilVax platform induces antigen-specific B- and T-cell responses.Immunol Cell Biol. 2021 Aug;99(7):767-781. doi: 10.1111/imcb.12462. Epub 2021 May 20. Immunol Cell Biol. 2021. PMID: 33866609
-
Intranasal delivery of nanoparticle-based vaccines.Ther Deliv. 2017 Jan;8(3):151-167. doi: 10.4155/tde-2016-0068. Ther Deliv. 2017. PMID: 28145824 Review.
-
Mucosal vaccines: a paradigm shift in the development of mucosal adjuvants and delivery vehicles.APMIS. 2015 Apr;123(4):275-88. doi: 10.1111/apm.12351. Epub 2015 Jan 29. APMIS. 2015. PMID: 25630573 Review.
Cited by
-
Microbial Delivery Vehicles for Allergens and Allergen-Derived Peptides in Immunotherapy of Allergic Diseases.Front Microbiol. 2018 Jul 2;9:1449. doi: 10.3389/fmicb.2018.01449. eCollection 2018. Front Microbiol. 2018. PMID: 30013543 Free PMC article. Review.
-
Pilus proteins from Streptococcus pyogenes stimulate innate immune responses through Toll-like receptor 2.Immunol Cell Biol. 2022 Mar;100(3):174-185. doi: 10.1111/imcb.12523. Epub 2022 Feb 24. Immunol Cell Biol. 2022. PMID: 35124861 Free PMC article.
-
Plasmid Replicons for the Production of Pharmaceutical-Grade pDNA, Proteins and Antigens by Lactococcus lactis Cell Factories.Int J Mol Sci. 2021 Jan 30;22(3):1379. doi: 10.3390/ijms22031379. Int J Mol Sci. 2021. PMID: 33573129 Free PMC article. Review.
-
Group A Streptococcus Pili-Roles in Pathogenesis and Potential for Vaccine Development.Microorganisms. 2024 Mar 11;12(3):555. doi: 10.3390/microorganisms12030555. Microorganisms. 2024. PMID: 38543606 Free PMC article. Review.
-
Harnessing the potential of Lactobacillus species for therapeutic delivery at the lumenal-mucosal interface.Future Sci OA. 2021 Feb 4;7(4):FSO671. doi: 10.2144/fsoa-2020-0153. Future Sci OA. 2021. PMID: 33815818 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

