Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments
- PMID: 29416335
- PMCID: PMC5790078
- DOI: 10.2147/IJN.S150897
Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments
Abstract
Objective: It is a great challenge to absorb and conduct biophysicochemical interactions at the nano-bio interface. Peptides are emerging as versatile materials whose function can be programmed to perform specific tasks. Peptides combined nanoparticles might be utilized as a new approach of treatment. Human β-defensin 3 (hBD3), possesses both antimicrobial and proregeneration properties. Gold nanoparticles (AuNPs) have shown promising applications in the field of tissue engineering. However, the coordinating effects of AuNPs and hBD3 on human periodontal ligament cells (hPDLCs) remain unknown. In this study, we systematically investigated whether AuNPs and hBD3 would be able to coordinate and enhance the osteogenic differentiation of hPDLCs in inflammatory microenvironments, and the underlying mechanisms was explored.
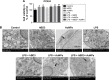
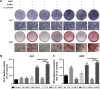
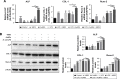
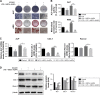
Methods: hPDLCs were stimulated with E. coli-LPS, hBD3 and AuNPs. Alkaline phosphatase (ALP) and alizarin red S staining were used to observe the effects of hBD3 and AuNPs on the osteogenic differentiation of hPDLCs. Real-time PCR and western blot were performed to evaluate the osteogenic differentiation and Wnt/β-catenin signaling pathway related gene and protein expression.
Results: In the inflammatory microenvironments stimulated by E. coli-LPS, we found that AuNPs and hBD3 increased the proliferation of hPDLCs slightly. In addition, hBD3-combined AuNPs could significantly enhance ALP activities and mineral deposition in vitro. Meanwhile, we observed that the osteogenic differentiation-related gene and protein expressions of ALP, collagenase-I (COL-1) and runt-related transcription factor 2 (Runx-2) were remarkably upregulated in the presence of hBD3 and AuNPs. Moreover, hBD3-combined AuNPs strongly activated the Wnt/β-catenin signaling pathway and upregulated the gene and protein expression of β-catenin and cyclin D1. Furthermore, hBD3-combined AuNPs induced osteogenesis, which could be reversed by the Wnt/β-catenin signaling pathway inhibitor (ICG-001).
Conclusion: The present study demonstrated that hBD3 combined AuNPs could significantly promote the osteogenic differentiation of hPDLCs in inflammatory microenvironments via activating the Wnt/β-catenin signaling pathway.
Keywords: Wnt/β-catenin signaling; gold nanoparticles; hBD3; inflammatory microenvironments; osteogenesis; periodontal ligament cells.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Hughes FJ, Ghuman M, Talal A. Periodontal regeneration: a challenge for the tissue engineer? Proc Inst Mech Eng H. 2010;224(12):1345–1358. - PubMed
-
- Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol. 2014;59(2):167–175. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

