Predictive factors for 6 vs 12 cycles of Folfiri-Bevacizumab in metastatic colorectal cancer
- PMID: 29416820
- PMCID: PMC5788688
- DOI: 10.18632/oncotarget.23355
Predictive factors for 6 vs 12 cycles of Folfiri-Bevacizumab in metastatic colorectal cancer
Abstract
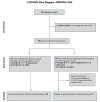
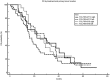
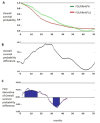
Early switching to de-intensified maintenance regimen is still a matter of debate in metastatic colorectal cancer (mCRC). The MARTHA trial, a S.I.C.O.G. phase III randomized trial, compared FOLOFIRI+bevacizumab (B) for 12 cycles (6 months) followed by B for up to 12 months (FOLFIRI +B*12 arm) vs FOLFIRI+B for 6 cycles (3 months) followed by capecitabine+B for 4 cycles followed by B for up to 12 months (FOLFIRI+B*6 arm). Chemotherapy-naïve mCRC patients were randomized, primary endpoint was progression free survival (PFS), with overall survival (OS) as a secondary endpoint. A novel analysis, the Death Pace Analysis (DPA), was performed to identify patients who benefited from a specific treatment. No PFS difference was seen in 198 enrolled patients (101 in FOLFIRI+B*12, 97 in FOLFIRI+B*6). A non-significant superior OS was observed for FOLFIRI+B*6 (HR 0.74, p 0.098). The DPA demonstrated that 14% of patients were identifiable as FOLFIRI+B*6-benefiting patients. According to a logistic regression analysis including 23 clinicopathological variables, baseline Hb was the only independent predictor of DPA-defined FOLFIRI+B*6-benefit status. Among patients with Hb ≤ 11.1 gr/dL a statistically significant prolonged OS was observed for FOLFIRI+B*6 over FOLFIRI+B*12 (median OS: 20.7 vs 12.6 months, respectively, HR 0.54, p 0.048). No survival difference was observed between arms in patients with Hb > 11.1. mCRC patients with low baseline Hb levels are better treated with FOLFIRI+B*6 first-line strategy. Possible biological explanations for this finding are being investigated.
Keywords: bevacizumab; death pace analysis; fluorouracil; irinotecan; metastatic colorectal cancer.
Conflict of interest statement
CONFLICTS OF INTERESTS No funding or conflicts of interests related to the present work have to be declared.
Figures


References
-
- Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–24. https://doi.org/10.1200/JCO.2014.59.7633. - DOI - PubMed
-
- Sanz-Garcia E, Grasselli J, Argiles G, Elez ME, Tabernero J. Current and advancing treatments for metastatic colorectal cancer. Expert Opin Biol Ther. 2016;16:93–110. https://doi.org/10.1517/14712598.2016.1108405. - DOI - PubMed
-
- Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C, Lindor NM, Minsky BD, Monzon FA, Sargent DJ, Singh VM, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453–86. https://doi.org/10.1200/JCO.2016.71.9807. - DOI - PubMed
-
- Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. 2017 https://doi.org/10.1093/annonc/mdx175. - DOI - PMC - PubMed
-
- Missiaglia E, Jacobs B, D’Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. https://doi.org/10.1093/annonc/mdu275. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

