Subversion of the Endocytic and Secretory Pathways by Bacterial Effector Proteins
- PMID: 29417046
- PMCID: PMC5787570
- DOI: 10.3389/fcell.2018.00001
Subversion of the Endocytic and Secretory Pathways by Bacterial Effector Proteins
Abstract
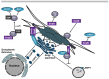
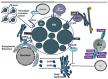
Intracellular bacteria have developed numerous strategies to hijack host vesicular trafficking pathways to form their unique replicative niches. To promote intracellular replication, the bacteria must interact with host organelles and modulate host signaling pathways to acquire nutrients and membrane for the growing parasitophorous vacuole all while suppressing activation of the immune response. To facilitate host cell subversion, bacterial pathogens use specialized secretion systems to deliver bacterial virulence factors, termed effectors, into the host cell that mimic, agonize, and/or antagonize the function of host proteins. In this review we will discuss how bacterial effector proteins from Coxiella burnetii, Brucella abortus, Salmonella enterica serovar Typhimurium, Legionella pneumophila, Chlamydia trachomatis, and Orientia tsutsugamushi manipulate the endocytic and secretory pathways. Understanding how bacterial effector proteins manipulate host processes not only gives us keen insight into bacterial pathogenesis, but also enhances our understanding of how eukaryotic membrane trafficking is regulated.
Keywords: Brucella; Chlamydia; Coxiella; Legionella; Orientia; Salmonella; secreted effector; vesicle trafficking.
Figures




References
-
- Aeberhard L., Banhart S., Fischer M., Jehmlich N., Rose L., Koch S., et al. . (2015). The proteome of the isolated Chlamydia trachomatis containing vacuole reveals a complex trafficking platform enriched for retromer components. PLoS Pathog. 11:e1004883. 10.1371/journal.ppat.1004883 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

