Inflammation in Vein Graft Disease
- PMID: 29417051
- PMCID: PMC5787541
- DOI: 10.3389/fcvm.2018.00003
Inflammation in Vein Graft Disease
Abstract
Bypass surgery is one of the most frequently used strategies to revascularize tissues downstream occlusive atherosclerotic lesions. For venous bypass surgery the great saphenous vein is the most commonly used vessel. Unfortunately, graft efficacy is low due to the development of vascular inflammation, intimal hyperplasia and accelerated atherosclerosis. Moreover, failure of grafts leads to significant adverse outcomes and even mortality. The last couple of decades not much has changed in the treatment of vein graft disease (VGD). However, insight is the cellular and molecular mechanisms of VGD has increased. In this review, we discuss the latest insights on VGD and the role of inflammation in this. We discuss vein graft pathophysiology including hemodynamic changes, the role of vessel wall constitutions and vascular remodeling. We show that profound systemic and local inflammatory responses, including inflammation of the perivascular fat, involve both the innate and adaptive immune system.
Keywords: atherosclerosis; bypass graft; cardiovascular disease; inflammation; innate immunity; saphenous vein; vein graft disease.
Figures
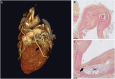
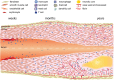
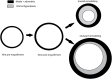
References
-
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J (2014) 35(37):2541–619.10.1093/eurheartj/ehu278 - DOI - PubMed
-
- Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol (2004) 44(11):2149–56.10.1016/j.jacc.2004.08.064 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

