A Novel Severity Assessment Scoring System for Hidradenitis Suppurativa
- PMID: 29417136
- PMCID: PMC5885841
- DOI: 10.1001/jamadermatol.2017.5890
A Novel Severity Assessment Scoring System for Hidradenitis Suppurativa
Abstract
Importance: The variation in both clinical appearance and responses to diverse treatment options emphasize the importance of an accurate, clinically relevant, yet easy-to-use scoring system in hidradenitis suppurativa.
Objective: To propose and provide validation data for the newly designed Severity Assessment of Hidradenitis Suppurativa score.
Design, setting, and participants: We prospectively assessed disease severity using Hurley staging and the modified Hidradenitis Suppurativa Score in 355 patients referred to Ruhr-University Bochum Department of Dermatology between March 2016 and June 2017. We also assessed disease severity via the Severity Assessment of Hidradenitis Suppurativa score.
Main outcomes and measures: Evaluation and assessment of convergent validity and responsiveness to treatment of the Severity Assessment of Hidradenitis Suppurativa score.
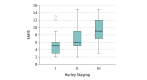
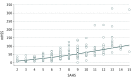
Results: Eighty-eight of the 355 patients (134 [37.7%] men and 221 [62.3%] women with a median [IQR] age of 40 [30-49] years) were classified as Hurley stage I, 221 were Hurley stage II, and 46 were Hurley stage III, with an overall median modified Hidradenitis Suppurativa Score of 31 (interquartile range [IQR], 19.3-53). The median total Severity Assessment of Hidradenitis Suppurativa score was 6 (IQR, 4-9), significantly different among the 3 Hurley groups. The median SAHS score for patients in Hurley stage I was 5 (IQR, 3-6), 6 (IQR, 5-9) for patients in Hurley stage II, and 9 (IQR, 7-12) for patients in Hurley stage III (P < .001, Kruskal-Wallis test). Correlation analysis showed a significant correlation between the modified Hidradenitis Suppurativa Score and the Severity Assessment of Hidradenitis Suppurativa score (r = 0.79, P < .001). Disease severity assessment before and after 3 months of conservative systemic treatment showed a significant correlation between the Severity Assessment of Hidradenitis Suppurativa score and modified Hidradenitis Suppurativa Score. Both the mHSS (P = .001) and the SAHS score (P < .001) significantly differed between the baseline visit (median mHSS, 33 [IQR, 24-52]; median SAHS score, 6 [IQR, 5-9]) and the 3-month visit (median mHSS, 28 [IQR, 15-43.5]; median SAHS score, 5 [IQR, 4-6.3]). The 2 patient-reported items demonstrated excellent test-retest reliability with intraclass correlation coefficient values greater than 0.8.
Conclusions and relevance: Our validation data demonstrated that the Severity Assessment of Hidradenitis Suppurativa score is a disease severity instrument that significantly correlates with Hurley staging and the modified Hidradenitis Suppurativa Score, and is responsive enough to measure treatment outcome.
Conflict of interest statement
Figures

Comment in
-
Distinguishing Mild, Moderate, and Severe Hidradenitis Suppurativa.JAMA Dermatol. 2018 Aug 1;154(8):971-972. doi: 10.1001/jamadermatol.2018.1599. JAMA Dermatol. 2018. PMID: 29926079 No abstract available.
-
Distinguishing Mild, Moderate, and Severe Hidradenitis Suppurativa-Reply.JAMA Dermatol. 2018 Aug 1;154(8):972. doi: 10.1001/jamadermatol.2018.1600. JAMA Dermatol. 2018. PMID: 30090941 No abstract available.
References
-
- Kirschke J, Hessam S, Bechara FG. [Hidradenitis suppurativa/acne inversa: an update]. Hautarzt. 2015;66(6):413-422. - PubMed
-
- Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231(2):184-190. - PubMed
-
- Lipsker D, Severac F, Freysz M, et al. The ABC of hidradenitis suppurativa: a validated glossary on how to name lesions. Dermatology. 2016;232(2):137-142. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

