Direct Oral Anticoagulants in Addition to Antiplatelet Therapy for Secondary Prevention After Acute Coronary Syndromes: A Systematic Review and Meta-analysis
- PMID: 29417147
- PMCID: PMC5885890
- DOI: 10.1001/jamacardio.2017.5306
Direct Oral Anticoagulants in Addition to Antiplatelet Therapy for Secondary Prevention After Acute Coronary Syndromes: A Systematic Review and Meta-analysis
Erratum in
-
Errors in Figure 3 and Affiliations.JAMA Cardiol. 2018 May 1;3(5):445. doi: 10.1001/jamacardio.2018.0643. JAMA Cardiol. 2018. PMID: 29590285 Free PMC article. No abstract available.
Abstract
Importance: Patients with acute coronary syndrome (ACS) remain at high risk for experiencing recurrent ischemic events. Direct oral anticoagulants (DOAC) have been proposed for secondary prevention after ACS.
Objective: To evaluate the safety and efficacy of DOAC in addition to antiplatelet therapy (APT) after ACS, focusing on treatment effects stratified by baseline clinical presentation (non-ST-segment elevation ACS [NSTE-ACS] vs ST-segment elevation myocardial infarction [STEMI]).
Data sources: PubMed, Embase, BioMedCentral, Google Scholar, and the Cochrane Central Register of Controlled Trials were searched from inception to March 1, 2017.
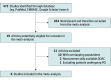
Study selection: Randomized clinical trials on DOAC after ACS were evaluated for inclusion. Overall, 473 studies were screened, 19 clinical trials were assessed as potentially eligible, and 6 were included in the meta-analysis.
Data extraction and synthesis: Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were used to abstract data and assess quality and validity. The risk of bias tool, version 2.0 (Cochrane) was used for risk of bias assessment. Data were pooled using random-effects models.
Main outcomes and measures: The prespecified primary efficacy end point was the composite of cardiovascular death, myocardial infarction, and stroke. The prespecified primary safety end point was major bleeding.
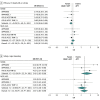
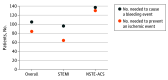
Results: Six trials that included 29 667 patients were identified (14 580 patients [49.1%] with STEMI and 15 036 [50.7%] with NSTE-ACS). The primary efficacy end point risk was significantly lower in patients who were treated with DOAC as compared with APT alone (odds ratio [OR], 0.85; 95% CI, 0.77-0.93; P < .001). This benefit was pronounced in patients with STEMI (OR, 0.76; 95% CI, 0.66-0.88; P < .001), while no significant treatment effect was observed in patients with NSTE-ACS (OR, 0.92; 95% CI, 0.78-1.09; P = .36; P for interaction = .09). With respect to safety, DOACs were associated with a higher risk of major bleeding as compared with APT alone (OR, 3.17; 95% CI, 2.27-4.42; P < .001), with consistent results in patients with STEMI (OR, 3.45; 95% CI, 1.95-6.09; P < .001) and NSTE-ACS (OR, 2.19; 95% CI, 1.38-3.48; P < .001; P for interaction = .23).
Conclusions and relevance: To our knowledge, these findings are the first evidence to support differential treatment effects of DOAC in addition to APT according to ACS baseline clinical presentation. In patients with NSTE-ACS, the risk-benefit profile of DOAC appears unfavorable. Conversely, DOAC in addition to APT might represent an attractive option for patients with STEMI.
Conflict of interest statement
Figures
References
-
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155-2165. - PubMed
-
- Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;2:2017.
-
- Roffi M, Patrono C, Collet J-P, et al. ; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. - PubMed
-
- Steg PG, James SK, Atar D, et al. ; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) . ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619. doi: 10.1093/eurheartj/ehs215 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

