Strain- and sex-dependent pulmonary toxicity of waterpipe smoke in mouse
- PMID: 29417753
- PMCID: PMC5803106
- DOI: 10.14814/phy2.13579
Strain- and sex-dependent pulmonary toxicity of waterpipe smoke in mouse
Abstract
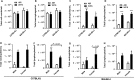
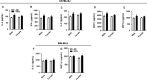
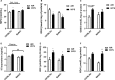
Waterpipe smoking is emerging as a form of tobacco smoking, but its lung health/risks is not known. It has been shown that different mouse strains show differences in susceptibility to tobacco smoke. However, the effect of waterpipe smoke (WPS) exposure and strain differences in susceptibility to oxidative and inflammatory responses is not known. Here, we showed acute WPS exposure induced oxidative stress and inflammatory response in C57BL/6J and BALB/cJ mouse strains. WPS exposure induced inflammatory cell influx (neutrophils and T-lymphocytes) in bronchoalveolar lavage fluid (BAL fluid), which varied among mouse strains. Proinflammatory cytokines release differed among both the strains, but was significantly increased in C57BL/6J mice. Myeloperoxidase levels in BAL fluid were increased significantly in both the strains. Total reduced glutathione (GSH) level was decreased, whereas the level of oxidized or glutathione disulfide (GSSG) increased in lungs of both the strains. Similarly, the level of lipid peroxidation markers, 15-isoprostane (plasma), malondialdehyde and 4-hydroxy-2-nonenal (lung homogenates) were increased by WPS. Our data suggest that, oxidative stress and inflammatory responses are influenced by strain characteristics during acute WPS exposure. Overall, C57BL/6J mice showed more susceptibility to oxidative stress and inflammatory responses compared to BALB/cJ mice. Acute WPS mediated pulmonary toxicity is differentially regulated in different mouse strains.
Keywords: Inflammation; glutathione; lipid peroxidation; oxidative stress; waterpipe (hookah).
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Bartalesi, B. , Cavarra E., Fineschi S., Lucattelli M., Lunghi B., Martorana P. A., et al. 2005. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur. Respir. J. 25:15–22. - PubMed
-
- Boskabady, M. H. , Farhang L., Mahmodinia M., Boskabady M., and Heydari G. R.. 2012. Comparison of pulmonary function and respiratory symptoms in water pipe and cigarette smokers. Respirology 17:950–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

