Enantioselective Synthesis of Trisubstituted Allenyl-B(pin) Compounds by Phosphine-Cu-Catalyzed 1,3-Enyne Hydroboration. Insights Regarding Stereochemical Integrity of Cu-Allenyl Intermediates
- PMID: 29417810
- PMCID: PMC6019291
- DOI: 10.1021/jacs.7b13296
Enantioselective Synthesis of Trisubstituted Allenyl-B(pin) Compounds by Phosphine-Cu-Catalyzed 1,3-Enyne Hydroboration. Insights Regarding Stereochemical Integrity of Cu-Allenyl Intermediates
Abstract
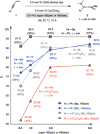
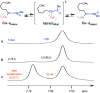
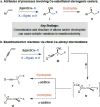
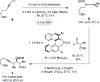
Catalytic enantioselective boron-hydride additions to 1,3-enynes, which afford allenyl-B(pin) (pin = pinacolato) products, are disclosed. Transformations are promoted by a readily accessible bis-phosphine-Cu complex and involve commercially available HB(pin). The method is applicable to aryl- and alkyl-substituted 1,3-enynes. Trisubstituted allenyl-B(pin) products were generated in 52-80% yield and, in most cases, in >98:2 allenyl:propargyl and 92:8-99:1 enantiomeric ratio. Utility is highlighted through a highly diastereoselective addition to an aldehyde, and a stereospecific catalytic cross-coupling process that delivers an enantiomerically enriched allene with three carbon-based substituents. The following key mechanistic attributes are elucidated: (1) Spectroscopic and computational investigations indicate that low enantioselectivity can arise from loss of kinetic stereoselectivity, which, as suggested by experimental evidence, may occur by formation of a propargylic anion generated by heterolytic Cu-C cleavage. This is particularly a problem when trapping of the Cu-allenyl intermediate is slow, namely, when an electron deficient 1,3-enyne or a less reactive boron-hydride reagent (e.g., HB(dan) (dan = naphthalene-1,8-diaminato)) is used or under non-optimal conditions (e.g., lower boron-hydride concentration causing slower trapping). (2) With enynes that contain a sterically demanding o-aryl substituent considerable amounts of the propargyl-B(pin) isomer may be generated (25-96%) because a less sterically demanding transition state for Cu/B exchange becomes favorable. (3) The phosphine ligand can promote isomerization of the enantiomerically enriched allenyl-B(pin) product; accordingly, lower ligand loading might at times be optimal. (4) Catalytic cross-coupling with an enantiomerically enriched allenyl-B(pin) compound might proceed with high stereospecificity (e.g., phosphine-Pd-catalyzed cross-coupling) or lead to considerable racemization (e.g., phosphine-Cu-catalyzed allylic substitution).
Figures









Similar articles
-
N-Heterocyclic Carbene-Copper-Catalyzed Group-, Site-, and Enantioselective Allylic Substitution with a Readily Accessible Propargyl(pinacolato)boron Reagent: Utility in Stereoselective Synthesis and Mechanistic Attributes.J Am Chem Soc. 2015 Jul 22;137(28):8948-64. doi: 10.1021/jacs.5b05805. Epub 2015 Jul 14. J Am Chem Soc. 2015. PMID: 26172476
-
SN2″-Selective and Enantioselective Substitution with Unsaturated Organoboron Compounds and Catalyzed by a Sulfonate-Containing NHC-Cu Complex.J Am Chem Soc. 2018 Dec 5;140(48):16842-16854. doi: 10.1021/jacs.8b10885. Epub 2018 Nov 16. J Am Chem Soc. 2018. PMID: 30444358
-
A broadly applicable NHC-Cu-catalyzed approach for efficient, site-, and enantioselective coupling of readily accessible (pinacolato)alkenylboron compounds to allylic phosphates and applications to natural product synthesis.J Am Chem Soc. 2014 Feb 5;136(5):2149-61. doi: 10.1021/ja4126565. Epub 2014 Jan 28. J Am Chem Soc. 2014. PMID: 24467274 Free PMC article.
-
Borylation and Stannylation Reactions with Tuning of Lewis Acidity.Chem Rec. 2021 Dec;21(12):3483-3497. doi: 10.1002/tcr.202100099. Epub 2021 Jun 1. Chem Rec. 2021. PMID: 34075689 Review.
-
Sulfonate N-Heterocyclic Carbene-Copper Complexes: Uniquely Effective Catalysts for Enantioselective Synthesis of C-C, C-B, C-H, and C-Si Bonds.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21304-21359. doi: 10.1002/anie.202003755. Epub 2020 Aug 26. Angew Chem Int Ed Engl. 2020. PMID: 32364640 Free PMC article. Review.
Cited by
-
Recent Advances in Catalytic Alkyne Transformation via Copper Carbene Intermediates.Molecules. 2022 May 11;27(10):3088. doi: 10.3390/molecules27103088. Molecules. 2022. PMID: 35630567 Free PMC article. Review.
-
A Pd-catalyzed highly selective three-component protocol for trisubstituted allenes.Chem Sci. 2023 Jun 22;14(28):7709-7715. doi: 10.1039/d3sc01849k. eCollection 2023 Jul 19. Chem Sci. 2023. PMID: 37476716 Free PMC article.
-
Synthesis of fluoroalkenes and fluoroenynes via cross-coupling reactions using novel multihalogenated vinyl ethers.Beilstein J Org Chem. 2024 Oct 24;20:2691-2703. doi: 10.3762/bjoc.20.226. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39469295 Free PMC article.
-
Asymmetric 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis.Nat Commun. 2022 Aug 26;13(1):5036. doi: 10.1038/s41467-022-32614-4. Nat Commun. 2022. PMID: 36028488 Free PMC article.
-
Asymmetric dihydroboration of allenes enabled by ligand relay catalysis.Nat Commun. 2024 Sep 18;15(1):8186. doi: 10.1038/s41467-024-51774-z. Nat Commun. 2024. PMID: 39294125 Free PMC article.
References
-
-
For representative reports, see:
- Lee Y, Hoveyda AH. J Am Chem Soc. 2009;131:3160–3161. - PMC - PubMed
- Lee Y, Jang H, Hoveyda AH. J Am Chem Soc. 2009;131:18234–18235. - PMC - PubMed
- Sasaki Y, Zhong C, Sawamura M, Ito H. J Am Chem Soc. 2010;132:1226–1227. - PubMed
- Jang H, Zhugralin AR, Lee Y, Hoveyda AH. J Am Chem Soc. 2011;133:7859–7871. - PubMed
- Corberán R, Mszar NW, Hoveyda AH. Angew Chem, Int Ed. 2011;50:7079–7082. - PubMed
- Sasaki Y, Horita Y, Zhong C, Sawamura M, Ito H. Angew Chem, Int Ed. 2011;50:2778–2782. - PubMed
- Meng F, Jang H, Hoveyda AH. Chem Eur J. 2013;19:3204–3214. - PubMed
- Kubota K, Yamamoto E, Ito H. Adv Synth Catal. 2013;355:3527–3531.
- Jang H, Jung B, Hoveyda AH. Org Lett. 2014;16:4658–4661. - PMC - PubMed
- Wang Z, He X, Zhang R, Zhang G, Xu G, Zhang Q, Xiong T, Zhang Q. Org Lett. 2017;19:3067–3070. - PubMed
-
-
-
For representative reports, see:
- Matsuda N, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2013;135:4934–4937. - PubMed
- Zhu S, Niljianskul N, Buchwald SL. J Am Chem Soc. 2013;135:15746–15749. - PMC - PubMed
- Miki Y, Hirano K, Satoh T, Miura M. Angew Chem, Int Ed. 2013;52:10830–10834. - PubMed
- Zhu S, Buchwald SL. J Am Chem Soc. 2014;136:15913–15916. - PMC - PubMed
- Shi SL, Buchwald SL. Nat Chem. 2015;7:38–44. - PMC - PubMed
- Sakae R, Hirano K, Satoh T, Miura M. Angew Chem, Int Ed. 2015;54:613–617. - PubMed
- Niljianskul N, Zhu S, Buchwald SL. Angew Chem, Int Ed. 2015;54:1638–1641. - PMC - PubMed
- Yang Y, Shi SL, Niu D, Liu P, Buchwald SL. Science. 2015;349:62–66. - PMC - PubMed
- Niu D, Buchwald SL. J Am Chem Soc. 2015;137:9716–9721. - PMC - PubMed
- Nishikawa D, Hirano K, Miura M. J Am Chem Soc. 2015;137:15620–15623. - PubMed
- Pirnot MT, Wang YM, Buchwald SL. Angew Chem, Int Ed. 2016;55:48–57. - PMC - PubMed
- Kato K, Hirano K, Miura M. Angew Chem, Int Ed. 2016;55:14400–14404. - PubMed
- Zhu S, Niljianskul N, Buchwald SL. Nat Chem. 2016;8:144–150. - PMC - PubMed
- Shi SL, Wong ZL, Buchwald SL. Nature. 2016;532:353–356. - PMC - PubMed
- Wang H, Yang JC, Buchwald SL. J Am Chem Soc. 2017;139:8428–8431. - PMC - PubMed
-
-
-
For representative reports, see:
- Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J Am Chem Soc. 2015;137:13760–13763. - PubMed
- Wang YM, Buchwald SL. J Am Chem Soc. 2016;138:5024–5027. - PMC - PubMed
- Han JT, Jang WJ, Kim N, Yun J. J Am Chem Soc. 2016;138:15146–15149. - PubMed
- Lee J, Torker S, Hoveyda AH. Angew Chem, Int Ed. 2017;56:821–826. - PMC - PubMed
- Radomkit S, Liu Z, Closs A, Mikus MS, Hoveyda AH. Tetrahedron. 2017;73:5011–5017. - PMC - PubMed
- Xu G, Zhao H, Fu B, Cang A, Zhang G, Zhang Q, Xiong T, Zhang Q. Angew Chem, Int Ed. 2017;56:13130–13134. - PubMed
- Lee J, Radomkit S, Torker S, del Pozo J, Hoveyda AH. Nat Chem. 2018;10:99–108. - PMC - PubMed
- Kim N, Han JT, Ryu DH, Yun J. Org Lett. 2017;19:6144–6147. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

