Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis
- PMID: 29419429
- PMCID: PMC5927982
- DOI: 10.3324/haematol.2017.182907
Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis
Abstract
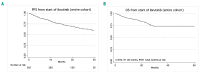
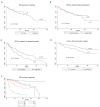
Clinical trials that led to ibrutinib's approval for the treatment of chronic lymphocytic leukemia showed that its side effects differ from those of traditional chemotherapy. Reasons for discontinuation in clinical practice have not been adequately studied. We conducted a retrospective analysis of chronic lymphocytic leukemia patients treated with ibrutinib either commercially or on clinical trials. We aimed to compare the type and frequency of toxicities reported in either setting, assess discontinuation rates, and evaluate outcomes. This multicenter, retrospective analysis included ibrutinib-treated chronic lymphocytic leukemia patients at nine United States cancer centers or from the Connect® Chronic Lymphocytic Leukemia Registry. We examined demographics, dosing, discontinuation rates and reasons, toxicities, and outcomes. The primary endpoint was progression-free survival. Six hundred sixteen ibrutinib-treated patients were identified. A total of 546 (88%) patients were treated with the commercial drug. Clinical trial patients were younger (mean age 58 versus 61 years, P=0.01) and had a similar time from diagnosis to treatment with ibrutinib (mean 85 versus 87 months, P=0.8). With a median follow-up of 17 months, an estimated 41% of patients discontinued ibrutinib (median time to ibrutinib discontinuation was 7 months). Notably, ibrutinib toxicity was the most common reason for discontinuation in all settings. The median progression-free survival and overall survival for the entire cohort were 35 months and not reached (median follow-up 17 months), respectively. In the largest reported series on ibrutinib- treated chronic lymphocytic leukemia patients, we show that 41% of patients discontinued ibrutinib. Intolerance as opposed to chronic lymphocytic leukemia progression was the most common reason for discontinuation. Outcomes remain excellent and were not affected by line of therapy or whether patients were treated on clinical studies or commercially. These data strongly argue in favor of finding strategies to minimize ibrutinib intolerance so that efficacy can be further maximized. Future clinical trials should consider time-limited therapy approaches, particularly in patients achieving a complete response, in order to minimize ibrutinib exposure.
Copyright © 2018 Ferrata Storti Foundation.
Figures
References
-
- O’Brien S, Jones J, Coutre S, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, openlabel, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. - PubMed
-
- Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

