Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients
- PMID: 29419657
- PMCID: PMC5908728
- DOI: 10.1097/j.pain.0000000000001171
Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients
Abstract
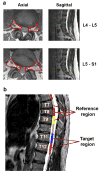
Numerous preclinical studies support the role of spinal neuroimmune activation in the pathogenesis of chronic pain, and targeting glia (eg, microglia/astrocyte)- or macrophage-mediated neuroinflammatory responses effectively prevents or reverses the establishment of persistent nocifensive behaviors in laboratory animals. However, thus far, the translation of those findings into novel treatments for clinical use has been hindered by the scarcity of data supporting the role of neuroinflammation in human pain. Here, we show that patients suffering from a common chronic pain disorder (lumbar radiculopathy), compared with healthy volunteers, exhibit elevated levels of the neuroinflammation marker 18 kDa translocator protein, in both the neuroforamina (containing dorsal root ganglion and nerve roots) and spinal cord. These elevations demonstrated a pattern of spatial specificity correlating with the patients' clinical presentation, as they were observed in the neuroforamen ipsilateral to the symptomatic leg (compared with both contralateral neuroforamen in the same patients as well as to healthy controls) and in the most caudal spinal cord segments, which are known to process sensory information from the lumbosacral nerve roots affected in these patients (compared with more superior segments). Furthermore, the neuroforaminal translocator protein signal was associated with responses to fluoroscopy-guided epidural steroid injections, supporting its role as an imaging marker of neuroinflammation, and highlighting the clinical significance of these observations. These results implicate immunoactivation at multiple levels of the nervous system as a potentially important and clinically relevant mechanism in human radicular pain, and suggest that therapies targeting immune cell activation may be beneficial for chronic pain patients.
Figures


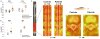

References
-
- Akaike H. Breakthroughs in statistics. Springer; 1992. Information theory and an extension of the maximum likelihood principle; pp. 610–624.
-
- Albrecht DS, Normandin MD, Shcherbinin S, Wooten DW, Schwarz AJ, Zurcher NR, Barth VN, Guehl NJ, Johnson-Akeju O, Atassi N, Veronese M, Turkheimer F, Hooker JM, Loggia ML. Pseudo-reference regions for glial imaging with 11C-PBR28: investigation in two clinical cohorts. J Nucl Med. 2017 - PMC - PubMed
-
- Aliyev A, Saboury B, Kwee TC, Torigian DA, Basu S, Wulff Christensen H, Alavi A. Age-related inflammatory changes in the spine as demonstrated by (18)F-FDG-PET:observation and insight into degenerative spinal changes. Hell J Nucl Med. 2012;15(3):197–201. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

