Exercise intolerance in Type 2 diabetes: is there a cardiovascular contribution?
- PMID: 29420147
- PMCID: PMC6008073
- DOI: 10.1152/japplphysiol.00070.2017
Exercise intolerance in Type 2 diabetes: is there a cardiovascular contribution?
Abstract
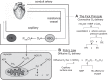
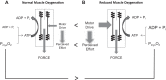
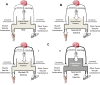
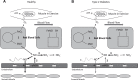
Physical activity is critically important for Type 2 diabetes management, yet adherence levels are poor. This might be partly due to disproportionate exercise intolerance. Submaximal exercise tolerance is highly sensitive to muscle oxygenation; impairments in exercising muscle oxygen delivery may contribute to exercise intolerance in Type 2 diabetes since there is considerable evidence for the existence of both cardiac and peripheral vascular dysfunction. While uncompromised cardiac output during submaximal exercise is consistently observed in Type 2 diabetes, it remains to be determined whether an elevated cardiac sympathetic afferent reflex could sympathetically restrain exercising muscle blood flow. Furthermore, while deficits in endothelial function are common in Type 2 diabetes and are often cited as impairing exercising muscle oxygen delivery, no direct evidence in exercise exists, and there are several other vasoregulatory mechanisms whose dysfunction could contribute. Finally, while there are findings of impaired oxygen delivery, conflicting evidence also exists. A definitive conclusion that Type 2 diabetes compromises exercising muscle oxygen delivery remains premature. We review these potentially dysfunctional mechanisms in terms of how they could impair oxygen delivery in exercise, evaluate the current literature on whether an oxygen delivery deficit is actually manifest, and correspondingly identify key directions for future research.
Keywords: cardiac dysfunction; exercise habits; microvascular flow; muscle blood flow; vasodilation.
Figures

References
-
- Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radic Biol Med 49: 1138–1144, 2010. doi: 10.1016/j.freeradbiomed.2010.06.033. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

