AP2σ Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity
- PMID: 29420171
- PMCID: PMC5792449
- DOI: 10.1016/j.celrep.2017.12.089
AP2σ Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity
Abstract
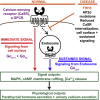
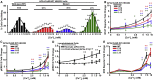
Spatial control of G-protein-coupled receptor (GPCR) signaling, which is used by cells to translate complex information into distinct downstream responses, is achieved by using plasma membrane (PM) and endocytic-derived signaling pathways. The roles of the endomembrane in regulating such pleiotropic signaling via multiple G-protein pathways remain unknown. Here, we investigated the effects of disease-causing mutations of the adaptor protein-2σ subunit (AP2σ) on signaling by the class C GPCR calcium-sensing receptor (CaSR). These AP2σ mutations increase CaSR PM expression yet paradoxically reduce CaSR signaling. Hypercalcemia-associated AP2σ mutations reduced CaSR signaling via Gαq/11 and Gαi/o pathways. The mutations also delayed CaSR internalization due to prolonged residency time of CaSR in clathrin structures that impaired or abolished endosomal signaling, which was predominantly mediated by Gαq/11. Thus, compartmental bias for CaSR-mediated Gαq/11 endomembrane signaling provides a mechanistic basis for multidimensional GPCR signaling.
Keywords: G proteins; GPCR; adaptor protein-2; calcium signaling; clathrin-mediated endocytosis; endosomal signaling; hypercalcemia.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Avlani V.A., Ma W., Mun H.C., Leach K., Delbridge L., Christopoulos A., Conigrave A.D. Calcium-sensing receptor-dependent activation of CREB phosphorylation in HEK293 cells and human parathyroid cells. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1097–E1104. - PubMed
-
- Chakravarti B., Chattopadhyay N., Brown E.M. Signaling through the extracellular calcium-sensing receptor (CaSR) In: Signaling C., Islam M.S., editors. Advances in Experimental Medicine and Biology. Springer; 2012. pp. 103–142. - PubMed
-
- Clague M.J., Urbé S. The interface of receptor trafficking and signalling. J. Cell Sci. 2001;114:3075–3081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

