New Insights Into the Role of mTOR Signaling in the Cardiovascular System
- PMID: 29420210
- PMCID: PMC6398933
- DOI: 10.1161/CIRCRESAHA.117.311147
New Insights Into the Role of mTOR Signaling in the Cardiovascular System
Abstract
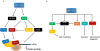
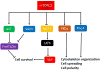
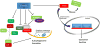
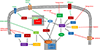
The mTOR (mechanistic target of rapamycin) is a master regulator of several crucial cellular processes, including protein synthesis, cellular growth, proliferation, autophagy, lysosomal function, and cell metabolism. mTOR interacts with specific adaptor proteins to form 2 multiprotein complexes, called mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2). In the cardiovascular system, the mTOR pathway regulates both physiological and pathological processes in the heart. It is needed for embryonic cardiovascular development and for maintaining cardiac homeostasis in postnatal life. Studies involving mTOR loss-of-function models revealed that mTORC1 activation is indispensable for the development of adaptive cardiac hypertrophy in response to mechanical overload. mTORC2 is also required for normal cardiac physiology and ensures cardiomyocyte survival in response to pressure overload. However, partial genetic or pharmacological inhibition of mTORC1 reduces cardiac remodeling and heart failure in response to pressure overload and chronic myocardial infarction. In addition, mTORC1 blockade reduces cardiac derangements induced by genetic and metabolic disorders and has been reported to extend life span in mice. These studies suggest that pharmacological targeting of mTOR may represent a therapeutic strategy to confer cardioprotection, although clinical evidence in support of this notion is still scarce. This review summarizes and discusses the new evidence on the pathophysiological role of mTOR signaling in the cardiovascular system.
Keywords: autophagy; cardiovascular diseases; heart; hypertrophy; mice.
© 2018 American Heart Association, Inc.
Conflict of interest statement
Figures

References
-
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–8. - PubMed
-
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. - PubMed
-
- Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

