Translational Implications of Platelets as Vascular First Responders
- PMID: 29420211
- PMCID: PMC5808568
- DOI: 10.1161/CIRCRESAHA.117.310939
Translational Implications of Platelets as Vascular First Responders
Abstract
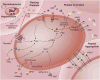
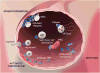
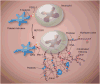
Platelets play a vital role in normal hemostasis to stem blood loss at sites of vascular injury by tethering and adhering to sites of injury, recruiting other platelets and blood cells to the developing clot, releasing vasoactive small molecules and proteins, and assembling and activating plasma coagulation proteins in a tightly regulated temporal and spatial manner. In synchrony with specific end products of coagulation, primarily cross-linked fibrin, a stable thrombus quickly forms. Far beyond physiological hemostasis and pathological thrombosis, emerging evidence supports platelets playing a pivotal role in vascular homeostasis, inflammation, cellular repair, regeneration, and wide range of autocrine and paracrine functions. In essence, platelets play both structural and functional roles as reporters, messengers, and active transporters surveying the vasculature for cues of environmental or developmental stimuli and participating as first responders.1 In this review, we will provide a contemporary perspective of platelet physiology, including fundamental, translational, and clinical constructs that apply directly to human health and disease.
Keywords: blood platelets; fibrin; humans; platelet activation; thrombosis.
© 2018 American Heart Association, Inc.
Figures
Similar articles
-
Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond.Crit Rev Clin Lab Sci. 2016 Dec;53(6):409-30. doi: 10.1080/10408363.2016.1200008. Epub 2016 Jul 22. Crit Rev Clin Lab Sci. 2016. PMID: 27282765 Review.
-
Platelet membrane glycoproteins in haemostasis.Clin Lab. 2002;48(5-6):247-62. Clin Lab. 2002. PMID: 12071575 Review.
-
[Platelets: biochemistry and physiology].Hamostaseologie. 2008 Dec;28(5):289-98. Hamostaseologie. 2008. PMID: 19132160 Review. German.
-
Hemostasis in the mouse (Mus musculus): a review.Thromb Haemost. 1999 Feb;81(2):177-88. Thromb Haemost. 1999. PMID: 10063988 Review. No abstract available.
-
An overview of hemostasis.Vet Clin North Am Small Anim Pract. 1988 Jan;18(1):5-20. doi: 10.1016/s0195-5616(88)50003-7. Vet Clin North Am Small Anim Pract. 1988. PMID: 3282384 Review.
Cited by
-
Platelet Protease Activated Receptor 1 Is Involved in the Hemostatic Effect of 20(S)-Protopanaxadiol by Regulating Calcium Signaling.Front Pharmacol. 2020 Sep 18;11:549150. doi: 10.3389/fphar.2020.549150. eCollection 2020. Front Pharmacol. 2020. PMID: 33041793 Free PMC article.
-
The Mechanisms of Restenosis and Relevance to Next Generation Stent Design.Biomolecules. 2022 Mar 10;12(3):430. doi: 10.3390/biom12030430. Biomolecules. 2022. PMID: 35327622 Free PMC article. Review.
-
Regenerative potential of PRP-based scaffolds in chronic wound healing: Mechanisms, advances, and therapeutic insights.Regen Ther. 2025 Jun 26;30:278-298. doi: 10.1016/j.reth.2025.06.008. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40654520 Free PMC article. Review.
-
The Effect of Extracellular Vesicles on Thrombosis.J Cardiovasc Transl Res. 2023 Jun;16(3):682-697. doi: 10.1007/s12265-022-10342-w. Epub 2022 Nov 28. J Cardiovasc Transl Res. 2023. PMID: 36441349 Free PMC article. Review.
-
Tethered platelet capture provides a mechanism for restricting circulating platelet activation to the wound site.Res Pract Thromb Haemost. 2023 Jan 23;7(2):100058. doi: 10.1016/j.rpth.2023.100058. eCollection 2023 Feb. Res Pract Thromb Haemost. 2023. PMID: 36865905 Free PMC article.
References
-
- Mancuso ME, Santagostino E. Platelets: Much more than bricks in a breached wall. Br J Haematol. 2017;178:209–219. - PubMed
-
- Schultze M. Ein heizbarer objecttisch und seine verwendung bei untersuchungen des blutes. Archiv für mikroscopische Anatomie. 1865;1:1–42.
-
- Bizzozero J. Ueber einen neuen forrnbestandteil des blutes und dessen rolle bei der thrombose und blutgerinnung. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1882;90:261–332.
-
- Wright JH. The histogenesis of thr blood platelets. J Morphology. 1910;21:263.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

