Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development
- PMID: 29420261
- PMCID: PMC6070131
- DOI: 10.1126/science.aal3589
Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development
Abstract
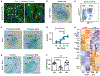
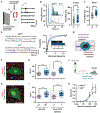
Neuronal synapse formation and remodeling are essential to central nervous system (CNS) development and are dysfunctional in neurodevelopmental diseases. Innate immune signals regulate tissue remodeling in the periphery, but how this affects CNS synapses is largely unknown. Here, we show that the interleukin-1 family cytokine interleukin-33 (IL-33) is produced by developing astrocytes and is developmentally required for normal synapse numbers and neural circuit function in the spinal cord and thalamus. We find that IL-33 signals primarily to microglia under physiologic conditions, that it promotes microglial synapse engulfment, and that it can drive microglial-dependent synapse depletion in vivo. These data reveal a cytokine-mediated mechanism required to maintain synapse homeostasis during CNS development.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

Comment in
-
Neural development: Instigating engulfment.Nat Rev Neurosci. 2018 May;19(5):253. doi: 10.1038/nrn.2018.31. Epub 2018 Mar 22. Nat Rev Neurosci. 2018. PMID: 29563573 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

