Enteric Immunity: Happy Gut, Healthy Animal
- PMID: 29421027
- PMCID: PMC7125775
- DOI: 10.1016/j.cvfa.2017.10.006
Enteric Immunity: Happy Gut, Healthy Animal
Abstract
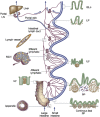
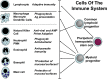
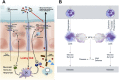
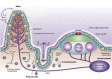
In this article, key concepts important for enteric immunity are discussed. The gastrointestinal tract is the largest immune organ of the body. The mucosal barrier, the tight junctions and the "kill zone," along with the gut mucosa and maintaining an "anti-inflammatory" state are essential for "good gut health." The microbiome, the microorganisms in the gastrointestinal tract, which has more cells then the entire animal's body, is essential for immune development, immune response, and maximizing ruminant productivity. Direct-fed microbials aid in both microbiome stability "homeostasis" and immune function.
Keywords: Bovine; Enteric; Immunology; Microbiome; Mucosal.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





References
-
- Charleston B., Fray M.D., Baigent S. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J Gen Virol. 2001;82:1893–1897. - PubMed
-
- Wilson R.A., Zonai A., Rudas P. T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Vet Immunol Immunopath. 1996;53:49–60. - PubMed
-
- Kampen A.H., Olsen I., Tollersrud T. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet Immunol Immunopath. 2006;113:53–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

