Multimodal Characterization of the Late Effects of Traumatic Brain Injury: A Methodological Overview of the Late Effects of Traumatic Brain Injury Project
- PMID: 29421973
- PMCID: PMC6016096
- DOI: 10.1089/neu.2017.5457
Multimodal Characterization of the Late Effects of Traumatic Brain Injury: A Methodological Overview of the Late Effects of Traumatic Brain Injury Project
Abstract
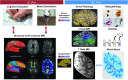
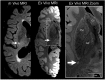
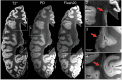
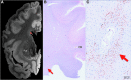
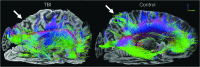
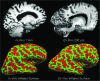
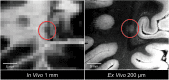
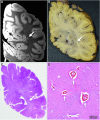
Epidemiological studies suggest that a single moderate-to-severe traumatic brain injury (TBI) is associated with an increased risk of neurodegenerative disease, including Alzheimer's disease (AD) and Parkinson's disease (PD). Histopathological studies describe complex neurodegenerative pathologies in individuals exposed to single moderate-to-severe TBI or repetitive mild TBI, including chronic traumatic encephalopathy (CTE). However, the clinicopathological links between TBI and post-traumatic neurodegenerative diseases such as AD, PD, and CTE remain poorly understood. Here, we describe the methodology of the Late Effects of TBI (LETBI) study, whose goals are to characterize chronic post-traumatic neuropathology and to identify in vivo biomarkers of post-traumatic neurodegeneration. LETBI participants undergo extensive clinical evaluation using National Institutes of Health TBI Common Data Elements, proteomic and genomic analysis, structural and functional magnetic resonance imaging (MRI), and prospective consent for brain donation. Selected brain specimens undergo ultra-high resolution ex vivo MRI and histopathological evaluation including whole-mount analysis. Co-registration of ex vivo and in vivo MRI data enables identification of ex vivo lesions that were present during life. In vivo signatures of postmortem pathology are then correlated with cognitive and behavioral data to characterize the clinical phenotype(s) associated with pathological brain lesions. We illustrate the study methods and demonstrate proof of concept for this approach by reporting results from the first LETBI participant, who despite the presence of multiple in vivo and ex vivo pathoanatomic lesions had normal cognition and was functionally independent until her mid-80s. The LETBI project represents a multidisciplinary effort to characterize post-traumatic neuropathology and identify in vivo signatures of postmortem pathology in a prospective study.
Keywords: MRI; dementia; neurodegeneration; neuropathology; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Dr. Fischl and Mr. Tirrell have financial interest in CorticoMetrics, a company whose medical pursuits focus on brain imaging and measurement technologies. Their interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Figures

References
-
- McKee A.C., Stein T.D., Nowinski C.J., Stern R.A., Daneshvar D.H., Alvarez V.E., Lee H.S., Hall G., Wojtowicz S.M., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 - PMC - PubMed
-
- Crane P.K., Gibbons L.E., Dams-O'Connor K., Trittschuh E., Leverenz J.B., Keene C.D., Sonnen J., Montine T.J., Bennett D.A., Leurgans S., Schneider J.A., and Larson E.B. (2016). Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 73, 1062–1069 - PMC - PubMed
-
- Health IOM COGWA (2008). Long-term consequences of traumatic brain injury. In: Gulf War and Health. National Academies Press: Washington, DC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EB023281/EB/NIBIB NIH HHS/United States
- R01 AG016495/AG/NIA NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- S10 RR019307/RR/NCRR NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- R01 AG008122/AG/NIA NIH HHS/United States
- R21 EB018907/EB/NIBIB NIH HHS/United States
- U54 HD083091/HD/NICHD NIH HHS/United States
- R01 EB006758/EB/NIBIB NIH HHS/United States
- K23 NS094538/NS/NINDS NIH HHS/United States
- R01 NS094003/NS/NINDS NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- R49 CE001171/CE/NCIPC CDC HHS/United States
- R01 EB019956/EB/NIBIB NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- K01 HD074651/HD/NICHD NIH HHS/United States
- R21 AG046657/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

