Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury
- PMID: 29421977
- PMCID: PMC5998829
- DOI: 10.1089/neu.2017.5399
Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury
Abstract
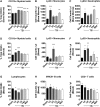
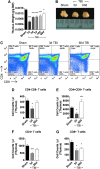
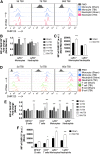
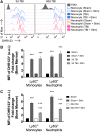
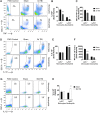
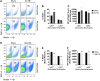
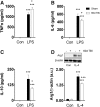
There is a compelling link between severe brain trauma and immunosuppression in patients with traumatic brain injury (TBI). Although acute changes in the systemic immune compartment have been linked to outcome severity, the long-term consequences of TBI on systemic immune function are unknown. Here, adult male C57Bl/6 mice underwent moderate-level controlled cortical impact (CCI) or sham surgery, and systemic immune function was evaluated at 1, 3, 7, 14, and 60 days post-injury. Bone marrow, blood, thymus, and spleen were examined by flow cytometry to assess changes in immune composition, reactive oxygen species (ROS) production, phagocytic activity, and cytokine production. Bone marrow derived macrophages (BMDMs) from sham and 60-day CCI mice were cultured for immune challenge studies using lipopolysaccharide (LPS) and interleukin-4 (IL-4) models. Acutely, TBI caused robust bone marrow activation and neutrophilia. Neutrophils and monocytes exhibited impairments in respiratory burst, cytokine production, and phagocytosis; in contrast, ROS levels and pro-inflammatory cytokine production were chronically elevated at 60 days post-injury. Cultures of BMDMs from chronic CCI mice demonstrated defects in LPS- and IL-4-induced polarization when compared with stimulated BMDMs from sham mice. TBI also caused thymic involution, inverted CD4:CD8 ratios, chronic T lymphopenia, greater memory conversion, increased T cell activation, impaired interferon γ induction, and chronically elevated Th1 cytokine and ROS production. Collectively, our in-depth phenotypic and functional analyses demonstrate that TBI induces widespread suppression of innate and adaptive immune responses after TBI. Moreover, at chronic time points, TBI mice exhibit hallmarks of accelerated immune aging, displaying chronic deficits in systemic immune function.
Keywords: chronic inflammation; immunosuppression; systemic immunity; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 - PubMed
-
- Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 - PubMed
-
- Alharfi I.M., Charyk Stewart T., Al Helali I., Daoud H., and Fraser D.D. (2014). Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J. Neurotrauma 31, 452–458 - PubMed
-
- Scott B.N., Roberts D.J., Robertson H.L., Kramer A.H., Laupland K.B., Ousman S.S., Kubes P., and Zygun D.A. (2013). Incidence, prevalence, and occurrence rate of infection among adults hospitalized after traumatic brain injury: study protocol for a systematic review and meta-analysis. Syst. Rev. 2, 68. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

