Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma
- PMID: 29422039
- PMCID: PMC5806286
- DOI: 10.1186/s12890-017-0554-8
Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma
Abstract
Background: Bronchial thermoplasty (BT) is a non-pharmacological intervention for severe asthma whose mechanism of action is not completely explained by a reduction of airway smooth muscle (ASM). In this study we analyzed the effect of BT on nerve fibers and inflammatory components in the bronchial mucosa at 1 year.
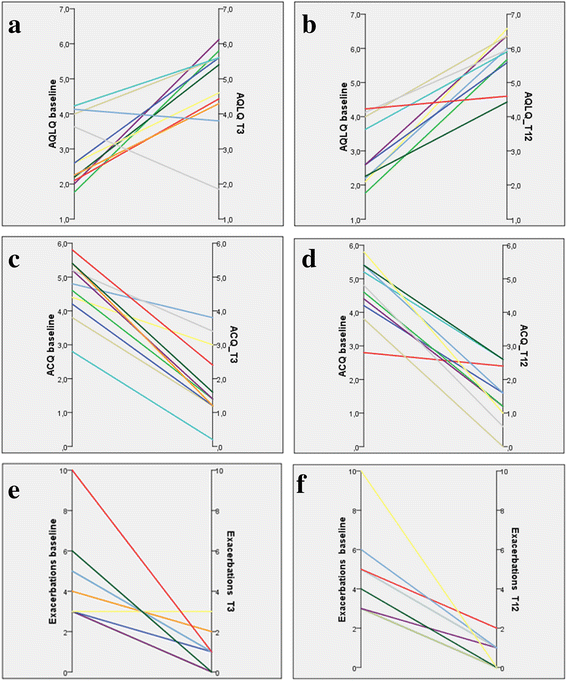
Methods: Endobronchial biopsies were obtained from 12 subjects (mean age 47 ± 11.3 years, 50% male) with severe asthma. Biopsies were performed at baseline (T0) and after 1 (T1), 2 (T2) and 12 (T12) months post-BT, and studied with immunocytochemistry and microscopy methods. Clinical data including Asthma Quality of Life Questionnaire (AQLQ) and Asthma Control Questionnaire (ACQ) scores, exacerbations, hospitalizations, oral corticosteroids use were also collected at the same time points.
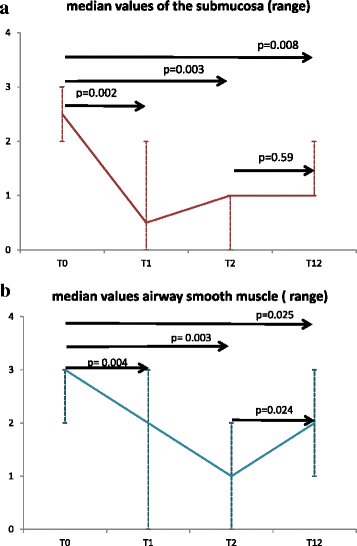
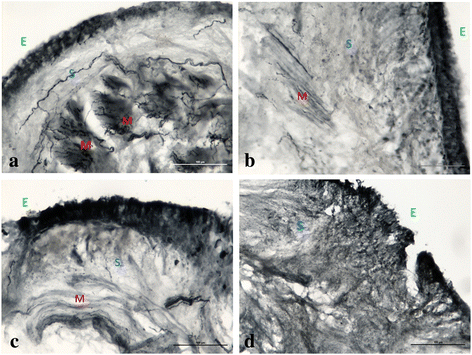
Results: A statistically significant reduction at T1, T2 and T12 of nerve fibers was observed in the submucosa and in ASM compared to T0. Among inflammatory cells, only CD68 showed significant changes at all time points. Improvement of all clinical outcomes was documented and persisted at the end of follow up.
Conclusions: A reduction of nerve fibers in epithelium and in ASM occurs earlier and persists at one year after BT. We propose that nerve ablation may contribute to mediate the beneficial effects of BT in severe asthma.
Trial registration: Registered on April 2, 2013 at ClinicalTrials.gov Identifier: NCT01839591 .
Keywords: Bronchial biopsies; Bronchial thermoplasty; Bronchoscopy; Nerve fibers; Severe asthma.
Conflict of interest statement
Ethics approval and consent to participate
The study conformed to the Declaration of Helsinki and was approved by the institutional ethics committee of Azienda Ospedaliera Arcispedale
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA). NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood. Institute. Available from www.ginasthma.org/. Updated 2012.
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. - DOI - PubMed
-
- Danek CJ, Lombard CM, Dungworth DL, Cox PG, Miller JD, Biggs MJ, Keast TM, Loomas BE, Wizeman WJ, Hogg JC, Leff AR. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97(5):1946–1953. doi: 10.1152/japplphysiol.01282.2003. - DOI - PubMed
-
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE. Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006. - PubMed
-
- Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D, Chaudhuri R, Miller JD, Laviolette MAIR. Trial study group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707. - DOI - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

