Hyperammonemia alters membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus by enhancing activation of the IL-1 receptor: underlying mechanisms
- PMID: 29422059
- PMCID: PMC5806265
- DOI: 10.1186/s12974-018-1082-z
Hyperammonemia alters membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus by enhancing activation of the IL-1 receptor: underlying mechanisms
Abstract
Background: Hyperammonemic rats reproduce the cognitive alterations of patients with hepatic encephalopathy, including altered spatial memory, attributed to altered membrane expression of AMPA receptor subunits in hippocampus. Neuroinflammation mediates these cognitive alterations. We hypothesized that hyperammonemia-induced increase in IL-1β in hippocampus would be responsible for the altered GluA1 and GluA2 membrane expression. The aims of this work were to (1) assess if increased IL-1β levels and activation of its receptor are responsible for the changes in GluA1 and/or GluA2 membrane expression in hyperammonemia and (2) identify the mechanisms by which activation of IL-1 receptor leads to altered membrane expression of GluA1 and GluA2.
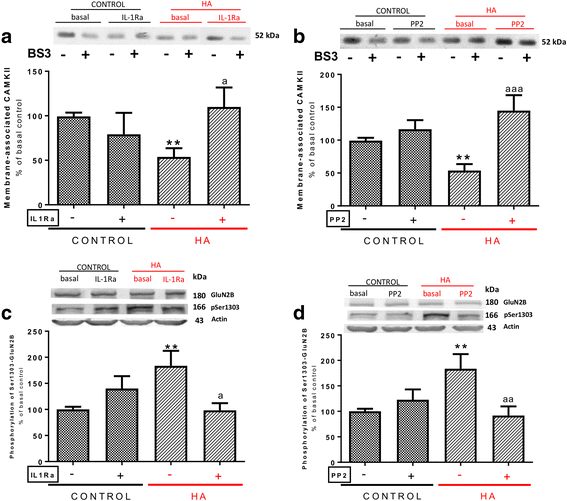
Methods: We analyzed in hippocampal slices from control and hyperammonemic rat membrane expression of AMPA receptors using the BS3 cross-linker and phosphorylation of the GluA1 and GluA2 subunits using phosphor-specific antibodies. The IL-1 receptor was blocked with IL-Ra, and the signal transduction pathways involved in modulation of membrane expression of GluA1 and GluA2 were analyzed using inhibitors of key steps.
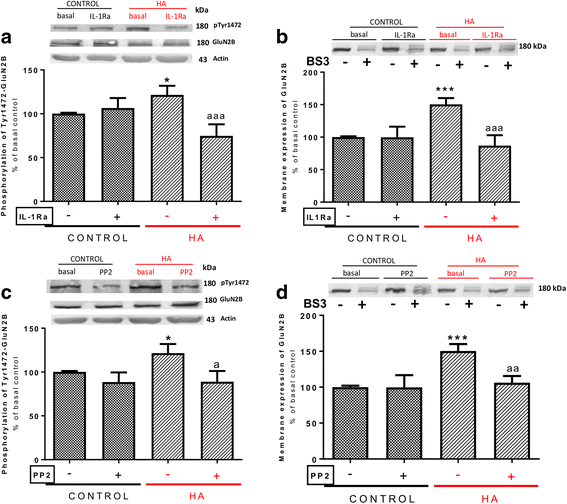
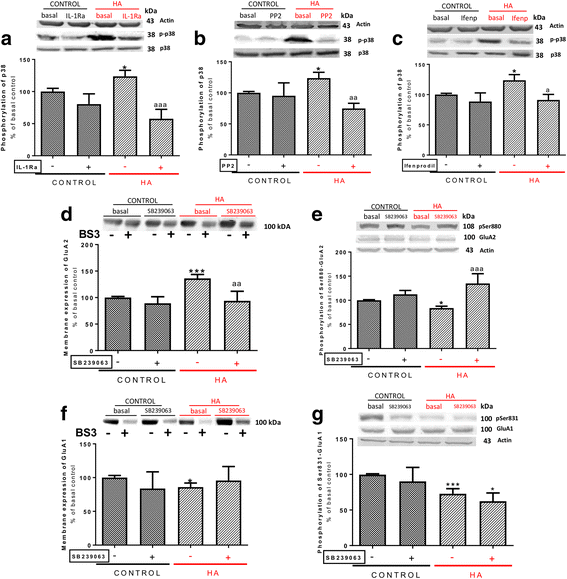
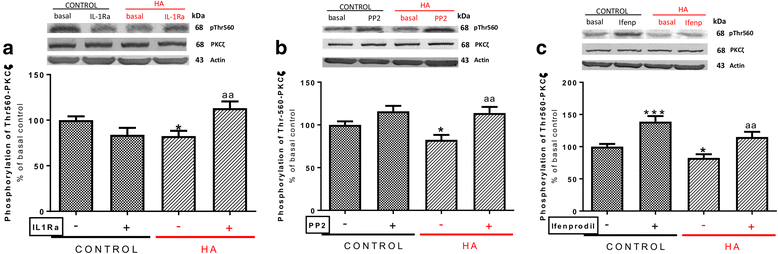
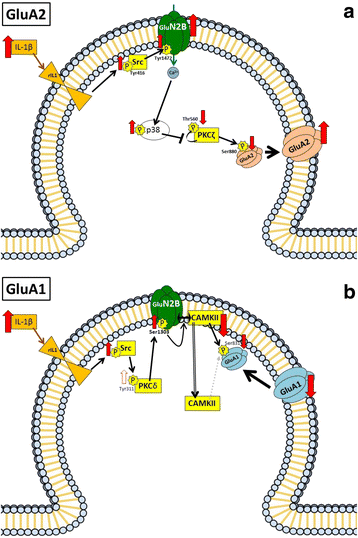
Results: Hyperammonemia reduces GluA1 and increases GluA2 membrane expression and reduces phosphorylation of GluA1 at Ser831 and of GluA2 at Ser880. Hyperammonemia increases IL-1β, enhancing activation of IL-1 receptor. This leads to activation of Src. The changes in membrane expression of GluA1 and GluA2 are reversed by blocking the IL-1 receptor with IL-1Ra or by inhibiting Src with PP2. After Src activation, the pathways for GluA2 and GluA1 diverge. Src increases phosphorylation of GluN2B at Tyr14721 and membrane expression of GluN2B in hyperammonemic rats, leading to activation of MAP kinase p38, which binds to and reduces phosphorylation at Thr560 and activity of PKCζ, resulting in reduced phosphorylation at Ser880 and enhanced membrane expression of GluA2. Increased Src activity in hyperammonemic rats also activates PKCδ which enhances phosphorylation of GluN2B at Ser1303, reducing membrane expression of CaMKII and phosphorylation at Ser831 and membrane expression of GluA1.
Conclusions: This work identifies two pathways by which neuroinflammation alters glutamatergic neurotransmission in hippocampus. The steps of the pathways identified could be targets to normalize neurotransmission in hyperammonemia and other pathologies associated with increased IL-1β by acting, for example, on p38 or PKCδ. IL-1β alters membrane expression of GluA1 and GluA2 AMPA receptor subunits by two difrerent mechanisms in the hippocampus of hyperammonemic rats.
Keywords: AMPA receptors; GluA1; GluA2; Hepatic encephalopathy; Hyperammonemia; IL-1 receptor; IL-1β; Membrane expression; Neuroinflammation; Neurotransmission.
Conflict of interest statement
Ethics approval and consent to participate
The experiments were approved by the Comite de Etica y Bienestar en Experimentacion Animal, Prince Felipe Research Center-Consellería de Agricultura, Generalitat Valenciana, and carried out in accordance with the European Communities Council Directive (86/609/EEC).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Extracellular cGMP Reverses Altered Membrane Expression of AMPA Receptors in Hippocampus of Hyperammonemic Rats: Underlying Mechanisms.Mol Neurobiol. 2019 Jun;56(6):4428-4439. doi: 10.1007/s12035-018-1387-z. Epub 2018 Oct 17. Mol Neurobiol. 2019. PMID: 30328550
-
Chronic hyperammonemia alters in opposite ways membrane expression of GluA1 and GluA2 AMPA receptor subunits in cerebellum. Molecular mechanisms involved.Biochim Biophys Acta Mol Basis Dis. 2018 Jan;1864(1):286-295. doi: 10.1016/j.bbadis.2017.10.031. Epub 2017 Oct 28. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29107806
-
In vivo administration of extracellular cGMP normalizes TNF-α and membrane expression of AMPA receptors in hippocampus and spatial reference memory but not IL-1β, NMDA receptors in membrane and working memory in hyperammonemic rats.Brain Behav Immun. 2016 Oct;57:360-370. doi: 10.1016/j.bbi.2016.05.011. Epub 2016 May 14. Brain Behav Immun. 2016. PMID: 27189036
-
Neuroinflammation alters GABAergic neurotransmission in hyperammonemia and hepatic encephalopathy, leading to motor incoordination. Mechanisms and therapeutic implications.Front Pharmacol. 2024 Mar 15;15:1358323. doi: 10.3389/fphar.2024.1358323. eCollection 2024. Front Pharmacol. 2024. PMID: 38560359 Free PMC article. Review.
-
Chronic hyperammonemia alters extracellular glutamate, glutamine and GABA and membrane expression of their transporters in rat cerebellum. Modulation by extracellular cGMP.Neuropharmacology. 2019 Dec 15;161:107496. doi: 10.1016/j.neuropharm.2019.01.011. Epub 2019 Jan 11. Neuropharmacology. 2019. PMID: 30641078 Review.
Cited by
-
Enhanced BDNF and TrkB Activation Enhance GABA Neurotransmission in Cerebellum in Hyperammonemia.Int J Mol Sci. 2022 Oct 4;23(19):11770. doi: 10.3390/ijms231911770. Int J Mol Sci. 2022. PMID: 36233065 Free PMC article.
-
Extracellular cGMP Reverses Altered Membrane Expression of AMPA Receptors in Hippocampus of Hyperammonemic Rats: Underlying Mechanisms.Mol Neurobiol. 2019 Jun;56(6):4428-4439. doi: 10.1007/s12035-018-1387-z. Epub 2018 Oct 17. Mol Neurobiol. 2019. PMID: 30328550
-
Bicuculline Reduces Neuroinflammation in Hippocampus and Improves Spatial Learning and Anxiety in Hyperammonemic Rats. Role of Glutamate Receptors.Front Pharmacol. 2019 Feb 25;10:132. doi: 10.3389/fphar.2019.00132. eCollection 2019. Front Pharmacol. 2019. PMID: 30858801 Free PMC article.
-
Extracellular vesicles from mesenchymal stem cells improve neuroinflammation and neurotransmission in hippocampus and cognitive impairment in rats with mild liver damage and minimal hepatic encephalopathy.Stem Cell Res Ther. 2024 Dec 18;15(1):472. doi: 10.1186/s13287-024-04076-6. Stem Cell Res Ther. 2024. PMID: 39696620 Free PMC article.
-
Hyperammonemia Enhances GABAergic Neurotransmission in Hippocampus: Underlying Mechanisms and Modulation by Extracellular cGMP.Mol Neurobiol. 2022 Jun;59(6):3431-3448. doi: 10.1007/s12035-022-02803-9. Epub 2022 Mar 23. Mol Neurobiol. 2022. PMID: 35320456
References
-
- Felipo V, Ordoño JF, Urios A, El Mlili N, Giménez-Garzó C, Aguado C, González-López O, Giner-Duran R, Serra MA, Abdallah W, Rodrigo JM, Salazar J, Montoliu C. Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology. 2012;5:530–539. doi: 10.1002/hep.24704. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

