Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding
- PMID: 29422079
- PMCID: PMC5806375
- DOI: 10.1186/s13071-018-2667-1
Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding
Abstract
Background: Ticks are obligate hematophagous parasites important economically and to health. Ticks consume large amounts of blood for their survival and reproduction; however, large amounts of iron in blood could lead to oxidative stress. Ticks use several molecules such as glutathione S-transferases (GSTs), ferritins, and peroxiredoxins to cope with oxidative stress. This study aimed to identify and characterize the GSTs of the hard tick Haemaphysalis longicornis in order to determine if they have a role in coping with oxidative stress.
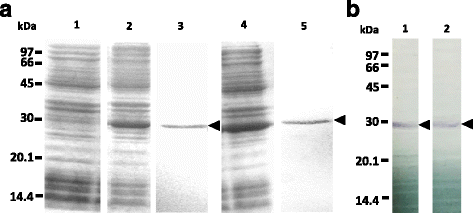
Methods: Genes encoding GSTs of H. longicornis were isolated from the midgut CDNA library. Genes have been cloned and recombinant GSTs have been expressed. The enzymatic activities, enzyme kinetic constants, and optimal pH of the recombinant GSTs toward 1-chloro-2,4-dinitrobenzene (CDNB) were determined. The gene transcription and protein expression profiles were determined in the whole ticks and internal organs, and developmental stages using real time RT-PCR and Western blotting during blood feeding. The localization of GST proteins in organs was also observed using immunofluorescent antibody test (IFAT).
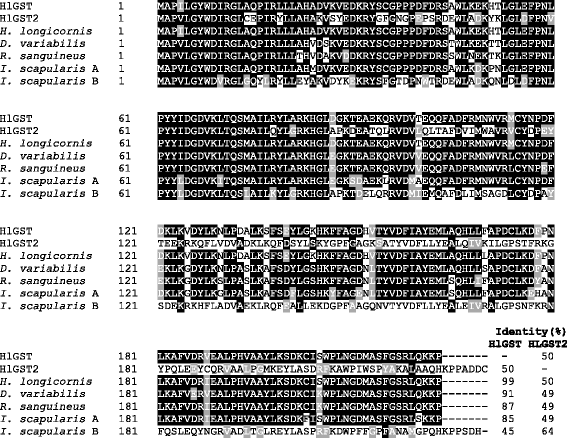
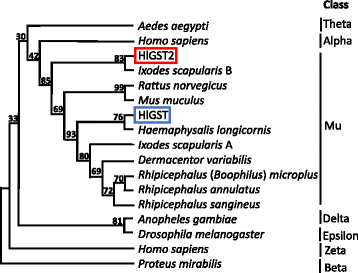
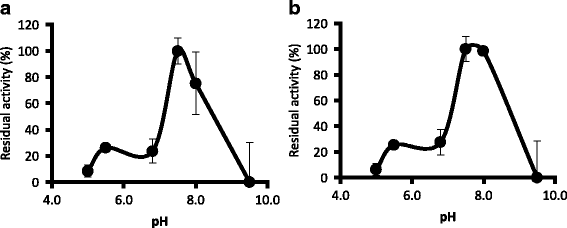
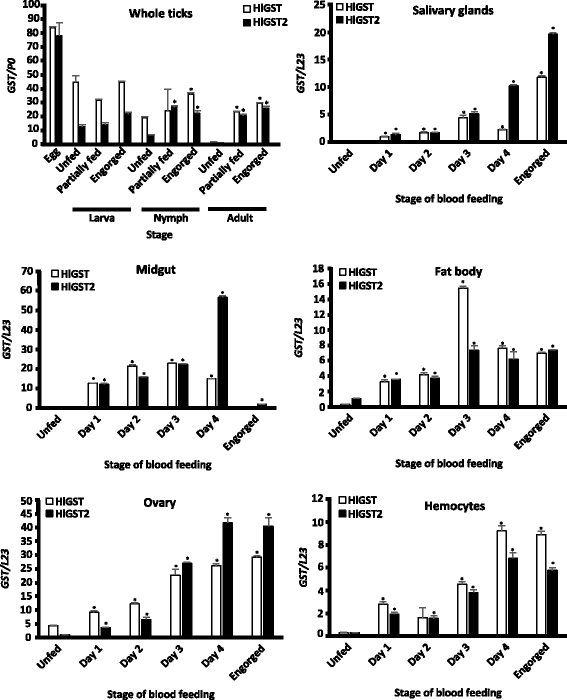
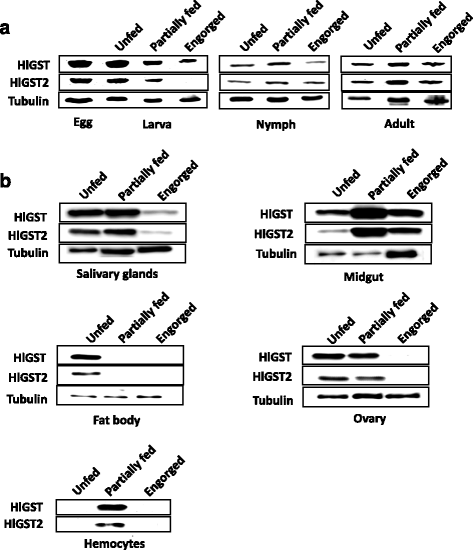
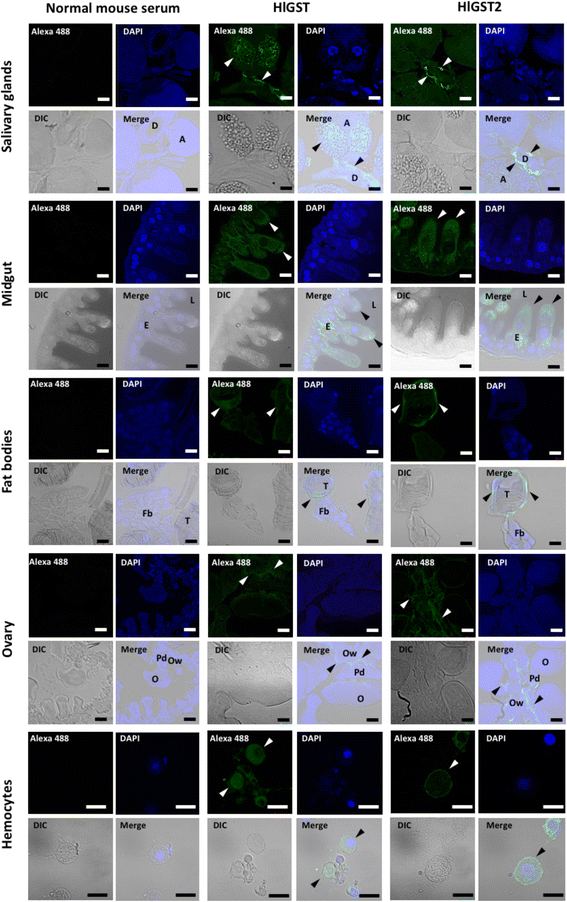
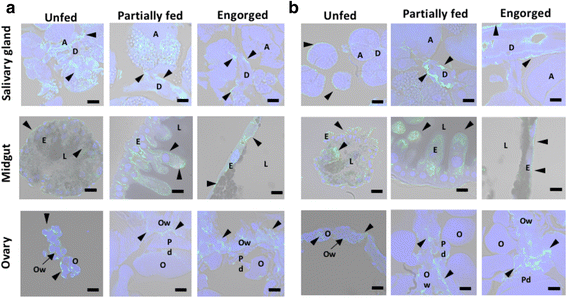
Results: We have isolated two genes encoding GSTs (HlGST and HlGST2). The enzymatic activity toward CDNB is 9.75 ± 3.04 units/mg protein for recombinant HlGST and 11.63 ± 4.08 units/mg protein for recombinant HlGST2. Kinetic analysis of recombinant HlGST showed K m values of 0.82 ± 0.14 mM and 0.64 ± 0.32 mM for the function of CDNB and GSH, respectively. Meanwhile, recombinant HlGST2 has K m values of 0.61 ± 0.20 mM and 0.53 ± 0.02 mM for the function of CDNB and GSH, respectively. The optimum pH of recombinant HlGST and recombinant HlGST2 activity was 7.5-8.0. Transcription of both GSTs increases in different developmental stages and organs during blood-feeding. GST proteins are upregulated during blood-feeding but decreased upon engorgement in whole ticks and in some organs, such as the midgut and hemocytes. Interestingly, salivary glands, ovaries, and fat bodies showed decreasing protein expression during blood-feeding to engorgement. Varying localization of GSTs in the midgut, salivary glands, fat bodies, ovaries, and hemocytes was observed depending on the feeding state, especially in the midgut and salivary glands.
Conclusions: In summary, a novel GST of H. longicornis has been identified. Characterization of the GSTs showed that GSTs have positive correlation with the degree and localization of oxidative stress during blood-feeding. This could indicate their protective role during oxidative stress.
Keywords: Blood-feeding; Glutathione S-transferases; Haemaphysalis longicornis; Oxidative stress; Tick.
Conflict of interest statement
Ethics approval and consent to participate
The care and use of experimental animals in this study were approved by the Animal Care and Use Committee of Kagoshima University (approval numbers VM15055 and VM15056 for the rabbits and mice, respectively).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Glutathione S-transferases play a role in the detoxification of flumethrin and chlorpyrifos in Haemaphysalis longicornis.Parasit Vectors. 2018 Aug 9;11(1):460. doi: 10.1186/s13071-018-3044-9. Parasit Vectors. 2018. PMID: 30092823 Free PMC article.
-
2-Cys peroxiredoxin is required in successful blood-feeding, reproduction, and antioxidant response in the hard tick Haemaphysalis longicornis.Parasit Vectors. 2016 Aug 19;9:457. doi: 10.1186/s13071-016-1748-2. Parasit Vectors. 2016. PMID: 27542835 Free PMC article.
-
Multiple ferritins are vital to successful blood feeding and reproduction of the hard tick Haemaphysalis longicornis.J Exp Biol. 2013 May 15;216(Pt 10):1905-15. doi: 10.1242/jeb.081240. Epub 2013 Feb 7. J Exp Biol. 2013. PMID: 23393286
-
Receptor for Advanced Glycation End Product (RAGE) Modulates Inflammation During Feeding of the Hard Tick, Haemaphysalis longicornis in Mice.Parasite Immunol. 2024 Jun;46(6):e13039. doi: 10.1111/pim.13039. Parasite Immunol. 2024. PMID: 38838041 Review.
-
The multiple roles of peroxiredoxins in tick blood feeding.Exp Appl Acarol. 2018 Jul;75(3):269-280. doi: 10.1007/s10493-018-0273-8. Epub 2018 Jul 20. Exp Appl Acarol. 2018. PMID: 30030662 Review.
Cited by
-
Low Genetic Polymorphism in the Immunogenic Sequences of Rhipicephalus microplus Clade C.Vaccines (Basel). 2022 Nov 11;10(11):1909. doi: 10.3390/vaccines10111909. Vaccines (Basel). 2022. PMID: 36423005 Free PMC article.
-
Depicting "arms race" of Rhipicephalus microplus and its host on a single frame platform.Parasitol Res. 2025 Feb 4;124(2):18. doi: 10.1007/s00436-025-08459-3. Parasitol Res. 2025. PMID: 39903310 Free PMC article.
-
Expression analysis of glutathione S-transferases and ferritins during the embryogenesis of the tick Haemaphysalis longicornis.Heliyon. 2020 Mar 30;6(3):e03644. doi: 10.1016/j.heliyon.2020.e03644. eCollection 2020 Mar. Heliyon. 2020. PMID: 32258487 Free PMC article.
-
The diverse functions of Mu-class Glutathione S-transferase HrGSTm1 during the development of Hyalomma rufipes with a focus on the detoxification metabolism of cyhalothrin.Parasit Vectors. 2024 Jan 2;17(1):1. doi: 10.1186/s13071-023-06084-6. Parasit Vectors. 2024. PMID: 38167098 Free PMC article.
-
From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness.Vaccines (Basel). 2021 Oct 15;9(10):1185. doi: 10.3390/vaccines9101185. Vaccines (Basel). 2021. PMID: 34696291 Free PMC article. Review.
References
-
- Freitas DRJ, Rosa RM, Moraes J, Campos E, Logullo C, Da Silva Vaz I, Masuda A. Relationship between glutathione S-transferase, catalase, oxygen consumption, lipid peroxidation and oxidative stress in eggs and larvae of Boophilus microplus (Acarina: Ixodidae) Comp Biochem Physiol, Part A Mol Integr Physiol. 2007;146:688–694. doi: 10.1016/j.cbpa.2006.04.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

