Incorporation of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG into Prostate Cancer Prevention Trial Risk Calculator
- PMID: 29422418
- PMCID: PMC6077104
- DOI: 10.1016/j.euf.2018.01.010
Incorporation of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG into Prostate Cancer Prevention Trial Risk Calculator
Abstract
Background: The Prostate Cancer Prevention Trial Risk Calculator (PCPTRC) is a commonly used risk tool for predicting the outcome on biopsy based on the established risk factors.
Objective: To determine whether incorporation of the novel urinary markers prostate cancer antigen 3 (PCA3) and TMPRSS2:ERG (T2:ERG) into the PCPTRC improves its discrimination, accuracy, and clinical net benefit.
Design, setting, and participants: Since PCA3 and T2:ERG were not measured as part of the PCPTRC, a Bayesian modeling approach was used to combine data where the markers were measured in a Michigan cohort with the PCPTRC as prior probabilities to form an updated PCPTRC. This update was compared to the existing PCPTRC on an independent Early Detection Research Network cohort in terms of discrimination, calibration, and decision curve analysis.
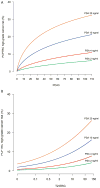
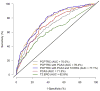
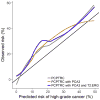
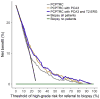
Results and limitations: Among the 1225 Michigan biopsies, 57.7%, 24.0%, and 18.3% were negative, with low- and high-grade (Gleason grade≥7) prostate cancer, respectively. Evaluated on the Early Detection Research Network validation set comprising 854 biopsies, areas under the curve (95% confidence interval) for predicting high-grade cancer in the 854 biopsies comprising the validation set were 70.0% (66.0-74.0%), 76.4% (72.8-80.0%), and 77.1% (73.6-80.6%) for the PCPTRC alone, with PCA3 added, and PCA3 and T2:ERG added, respectively. Net benefit was improved for the updated PCPTRC, while calibration was not. Limitations are that the updated PCPTRC is based on two different cohorts, the PCPT and Michigan, and that 20% of the validation set came from the Michigan center. More validation is required; hence, the updated risk tool is posted online.
Conclusions: Incorporation of PCA3 into the PCPTRC improved validation on an independent cohort, whereas T2:ERG offered negligible utility in addition to PCA3.
Patient summary: After passing external validation, prostate cancer antigen 3 has been added to the online Prostate Cancer Prevention Trial Risk Calculator for use by patients in deciding whether to proceed to biopsy. TMPRSS2:ERG did not improve prediction on the external validation set, but is included for further validation.
Keywords: PCA3; Prostate cancer; Prostate-specific antigen; T2:ERG.
Published by Elsevier B.V.
Figures
Similar articles
-
Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment.Eur Urol. 2016 Jul;70(1):45-53. doi: 10.1016/j.eururo.2015.04.039. Epub 2015 May 16. Eur Urol. 2016. PMID: 25985884 Free PMC article.
-
Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer.Eur Urol. 2014 Mar;65(3):534-42. doi: 10.1016/j.eururo.2012.11.014. Epub 2012 Nov 15. Eur Urol. 2014. PMID: 23201468
-
Performance of PCA3 and TMPRSS2:ERG urinary biomarkers in prediction of biopsy outcome in the Canary Prostate Active Surveillance Study (PASS).Prostate Cancer Prostatic Dis. 2019 Sep;22(3):438-445. doi: 10.1038/s41391-018-0124-z. Epub 2019 Jan 21. Prostate Cancer Prostatic Dis. 2019. PMID: 30664734 Free PMC article.
-
Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions.Prostate Cancer Prostatic Dis. 2017 Mar;20(1):12-19. doi: 10.1038/pcan.2016.59. Epub 2016 Dec 6. Prostate Cancer Prostatic Dis. 2017. PMID: 27922627 Review.
-
Prostate Cancer Detection and Prognosis: From Prostate Specific Antigen (PSA) to Exosomal Biomarkers.Int J Mol Sci. 2016 Oct 26;17(11):1784. doi: 10.3390/ijms17111784. Int J Mol Sci. 2016. PMID: 27792187 Free PMC article. Review.
Cited by
-
Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis.Int J Mol Sci. 2019 Apr 2;20(7):1637. doi: 10.3390/ijms20071637. Int J Mol Sci. 2019. PMID: 30986955 Free PMC article. Review.
-
How to Integrate Prostate Cancer Biomarkers in Urology Clinical Practice: An Update.Cancers (Basel). 2024 Jan 11;16(2):316. doi: 10.3390/cancers16020316. Cancers (Basel). 2024. PMID: 38254807 Free PMC article. Review.
-
Prostate cancer biomarkers and multiparametric MRI: is there a role for both in prostate cancer management?Ther Adv Urol. 2021 Mar 2;13:1756287221997186. doi: 10.1177/1756287221997186. eCollection 2021 Jan-Dec. Ther Adv Urol. 2021. PMID: 33737957 Free PMC article. Review.
-
Liquid Biomarkers in Prostate Cancer Diagnosis: Current Status and Emerging Prospects.World J Mens Health. 2025 Jan;43(1):8-27. doi: 10.5534/wjmh.230386. Epub 2024 Apr 11. World J Mens Health. 2025. PMID: 38772530 Free PMC article. Review.
-
Unravelling the Role of P300 and TMPRSS2 in Prostate Cancer: A Literature Review.Int J Mol Sci. 2023 Jul 11;24(14):11299. doi: 10.3390/ijms241411299. Int J Mol Sci. 2023. PMID: 37511059 Free PMC article. Review.
References
-
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–34. - PubMed
-
- Auffenberg GB, Merdan S, Miller DC, et al. Evaluation of prostate cancer risk calculators for shared decision making across diverse urology practices in Michigan. Urology. 2017;104:137–42. - PubMed
-
- Poyet C, Nieboer D, Bhindi B, et al. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int. 2016;117:401–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA138627/CA/NCI NIH HHS/United States
- U01 CA182883/CA/NCI NIH HHS/United States
- U01 CA214170/CA/NCI NIH HHS/United States
- U01 CA086402/CA/NCI NIH HHS/United States
- L30 CA199569/CA/NCI NIH HHS/United States
- P20 CA165589/CA/NCI NIH HHS/United States
- R01 GM070335/GM/NIGMS NIH HHS/United States
- U24 CA086368/CA/NCI NIH HHS/United States
- R01 CA179115/CA/NCI NIH HHS/United States
- R03 HS024810/HS/AHRQ HHS/United States
- R01 CA074015/CA/NCI NIH HHS/United States
- U01 CA113913/CA/NCI NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- T32 CA106209/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

