Pallidal deep brain stimulation modulates excessive cortical high β phase amplitude coupling in Parkinson disease
- PMID: 29422442
- PMCID: PMC5930048
- DOI: 10.1016/j.brs.2018.01.028
Pallidal deep brain stimulation modulates excessive cortical high β phase amplitude coupling in Parkinson disease
Abstract
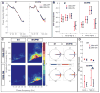
Objective: Deep brain stimulation (DBS) of the subthalamic nucleus (STN) and globus pallidus internus (GPi) are equally efficacious in the management of Parkinson disease (PD). Studies of STN-DBS have revealed a therapeutic reduction in excessive cortical β-γ phase-amplitude coupling (PAC). It is unclear whether this is specific to STN-DBS and potentially mediated by modulation of the hyperdirect pathway or if it is a generalizable mechanism seen with DBS of other targets. Moreover, it remains unclear how cortical signals are differentially modulated by movement versus therapy. To clarify, the effects of GPi-DBS and movement on cortical β power and β-γ PAC were examined.
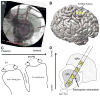
Methods: Right sensorimotor electrocorticographic signals were recorded in 10 PD patients undergoing GPi-DBS implantation surgery. We evaluated cortical β power and β-γ PAC during blocks of rest and contralateral hand movement (finger tapping) with GPi-DBS off and on.
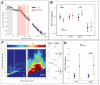
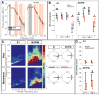
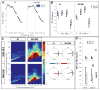
Results: Movement suppressed cortical low β power (P = 0.008) and high β-γ PAC (P = 0.028). Linear mixed effect modeling (LMEM) showed that power in low and high β bands are differentially modulated by movement (P = 0.022). GPi-DBS also results in a significant suppression of high β-γ PAC but without power modulation in either β sub-band (P = 0.008). Cortical high β-γ PAC is significantly correlated with severity of bradykinesia (Rho = 0.59, P = 0.045) and changes proportionally with therapeutic improvement (Rho = 0.61, P = 0.04).
Conclusions: Similar to STN-DBS, GPi-DBS reduces motor cortical β-γ PAC, like that also reported with dopaminergic mediations, suggesting it is a generalizable symptom biomarker in PD, independent of therapeutic target or proximity to the hyperdirect pathway.
Keywords: Deep brain stimulation; Globus pallidus internus; Motor cortex; Parkinson disease; Phase amplitude coupling.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Parkinsonian Beta Dynamics during Rest and Movement in the Dorsal Pallidum and Subthalamic Nucleus.J Neurosci. 2020 Apr 1;40(14):2859-2867. doi: 10.1523/JNEUROSCI.2113-19.2020. Epub 2020 Feb 27. J Neurosci. 2020. PMID: 32107277 Free PMC article.
-
Pallidal low β-low γ phase-amplitude coupling inversely correlates with Parkinson disease symptoms.Clin Neurophysiol. 2017 Nov;128(11):2165-2178. doi: 10.1016/j.clinph.2017.08.001. Epub 2017 Sep 5. Clin Neurophysiol. 2017. PMID: 28942154 Free PMC article.
-
Pallidal stimulation in Parkinson disease differentially modulates local and network β activity.J Neural Eng. 2018 Oct;15(5):056016. doi: 10.1088/1741-2552/aad0fb. Epub 2018 Jul 4. J Neural Eng. 2018. PMID: 29972146 Free PMC article.
-
Cognition and Depression Following Deep Brain Stimulation of the Subthalamic Nucleus and Globus Pallidus Pars Internus in Parkinson's Disease: A Meta-Analysis.Neuropsychol Rev. 2015 Dec;25(4):439-54. doi: 10.1007/s11065-015-9302-0. Epub 2015 Oct 12. Neuropsychol Rev. 2015. PMID: 26459361 Review.
-
Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations.Mov Disord. 2017 Jan;32(1):80-88. doi: 10.1002/mds.26837. Epub 2016 Nov 8. Mov Disord. 2017. PMID: 27859579 Review.
Cited by
-
Neural responses to social decision-making in suicide attempters with mental disorders.BMC Psychiatry. 2023 Jan 9;23(1):19. doi: 10.1186/s12888-022-04422-z. BMC Psychiatry. 2023. PMID: 36624426 Free PMC article.
-
Movement-Modulation of Local Power and Phase Amplitude Coupling in Bilateral Globus Pallidus Interna in Parkinson Disease.Front Hum Neurosci. 2018 Jul 9;12:270. doi: 10.3389/fnhum.2018.00270. eCollection 2018. Front Hum Neurosci. 2018. PMID: 30038563 Free PMC article.
-
Cross-frequency phase-amplitude coupling in repetitive movements in patients with Parkinson's disease.J Neurophysiol. 2022 Jun 1;127(6):1606-1621. doi: 10.1152/jn.00541.2021. Epub 2022 May 11. J Neurophysiol. 2022. PMID: 35544757 Free PMC article.
-
Closed-Loop Adaptive Deep Brain Stimulation in Parkinson's Disease: Procedures to Achieve It and Future Perspectives.J Parkinsons Dis. 2023;13(4):453-471. doi: 10.3233/JPD-225053. J Parkinsons Dis. 2023. PMID: 37182899 Free PMC article. Review.
-
High-Gamma Activity Is Coupled to Low-Gamma Oscillations in Precentral Cortices and Modulates with Movement and Speech.eNeuro. 2024 Feb 14;11(2):ENEURO.0163-23.2023. doi: 10.1523/ENEURO.0163-23.2023. Print 2024 Feb. eNeuro. 2024. PMID: 38242691 Free PMC article.
References
-
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002;125:1196–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

