TGFβ1 reinforces arterial aging in the vascular smooth muscle cell through a long-range regulation of the cytoskeletal stiffness
- PMID: 29422510
- PMCID: PMC5805716
- DOI: 10.1038/s41598-018-20763-w
TGFβ1 reinforces arterial aging in the vascular smooth muscle cell through a long-range regulation of the cytoskeletal stiffness
Abstract
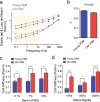
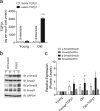
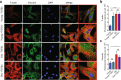
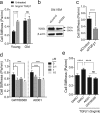
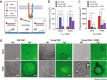
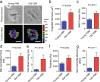
Here we report exquisitely distinct material properties of primary vascular smooth muscle (VSM) cells isolated from the thoracic aorta of adult (8 months) vs. aged (30 months) F344XBN rats. Individual VSM cells derived from the aged animals showed a tense internal network of the actin cytoskeleton (CSK), exhibiting increased stiffness (elastic) and frictional (loss) moduli than those derived from the adult animals over a wide frequency range of the imposed oscillatory deformation. This discrete mechanical response was long-lived in culture and persistent across a physiological range of matrix rigidity. Strikingly, the pro-fibrotic transforming growth factor β1 (TGFβ1) emerged as a specific modifier of age-associated VSM stiffening in vitro. TGFβ1 reinforced the mechanical phenotype of arterial aging in VSM cells on multiple time and length scales through clustering of mechanosensitive α5β1 and αvβ3 integrins. Taken together, these studies identify a novel nodal point for the long-range regulation of VSM stiffness and serve as a proof-of-concept that the broad-based inhibition of TGFβ1 expression, or TGFβ1 signal transduction in VSM, may be a useful therapeutic approach to mitigate the pathologic progression of central arterial wall stiffening associated with aging.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

