Poly(rC) binding protein 2 acts as a negative regulator of IRES-mediated translation of Hr mRNA
- PMID: 29422543
- PMCID: PMC5903819
- DOI: 10.1038/emm.2017.262
Poly(rC) binding protein 2 acts as a negative regulator of IRES-mediated translation of Hr mRNA
Abstract
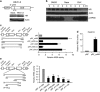
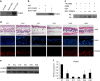
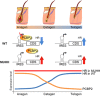
During the hair follicle (HF) cycle, HR protein expression is not concordant with the presence of the Hr mRNA transcript, suggesting an elaborate regulation of Hr gene expression. Here we present evidence that the 5' untranslated region (UTR) of the Hr gene has internal ribosome entry site (IRES) activity and this activity is regulated by the binding of poly (rC) binding protein 2 (PCBP2) to Hr mRNA. Overexpression and knockdown of PCBP2 resulted in a decrease in Hr 5' UTR IRES activity and an increase in HR protein expression without changing mRNA levels. We also found that this regulation was disrupted in a mutant Hr 5' UTR that has a mutation responsible for Marie Unna hereditary hypotrichosis (MUHH) in both mice and humans. These findings suggest that Hr mRNA expression is regulated at the post-transcriptional level via IRES-mediated translation control through interaction with PCPB2, but not in MUHH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nuclear Protein Sam68 Interacts with the Enterovirus 71 Internal Ribosome Entry Site and Positively Regulates Viral Protein Translation.J Virol. 2015 Oct;89(19):10031-43. doi: 10.1128/JVI.01677-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202240 Free PMC article.
-
Differential utilization of poly(rC) binding protein 2 in translation directed by picornavirus IRES elements.RNA. 1999 Dec;5(12):1570-85. doi: 10.1017/s1355838299991483. RNA. 1999. PMID: 10606268 Free PMC article.
-
Annexin A2 binds the internal ribosomal entry site of c-myc mRNA and regulates its translation.RNA Biol. 2021 Oct 15;18(sup1):337-354. doi: 10.1080/15476286.2021.1947648. Epub 2021 Aug 4. RNA Biol. 2021. PMID: 34346292 Free PMC article.
-
Targeting IRES-Mediated p53 Synthesis for Cancer Diagnosis and Therapeutics.Int J Mol Sci. 2017 Jan 4;18(1):93. doi: 10.3390/ijms18010093. Int J Mol Sci. 2017. PMID: 28054974 Free PMC article. Review.
-
Regulation of translation by specific protein/mRNA interactions.Biochimie. 1994;76(9):867-79. doi: 10.1016/0300-9084(94)90189-9. Biochimie. 1994. PMID: 7880904 Review.
Cited by
-
Poly(C)-binding Protein 2 Regulates the p53 Expression via Interactions with the 5'-Terminal Region of p53 mRNA.Int J Mol Sci. 2021 Dec 10;22(24):13306. doi: 10.3390/ijms222413306. Int J Mol Sci. 2021. PMID: 34948101 Free PMC article.
-
Poly(rC)-Binding Protein 2 Does Not Directly Participate in HCV Translation or Replication, but Rather Modulates Genome Packaging.Viruses. 2024 Jul 30;16(8):1220. doi: 10.3390/v16081220. Viruses. 2024. PMID: 39205194 Free PMC article.
-
PCBP2 in gastrointestinal cancers: fundamental mechanism and clinical potential.J Mol Med (Berl). 2025 Jul;103(7):779-794. doi: 10.1007/s00109-025-02562-9. Epub 2025 Jun 7. J Mol Med (Berl). 2025. PMID: 40481884 Review.
-
Targeting IRES-dependent translation as a novel approach for treating Duchenne muscular dystrophy.RNA Biol. 2021 Sep;18(9):1238-1251. doi: 10.1080/15476286.2020.1847894. Epub 2020 Nov 19. RNA Biol. 2021. PMID: 33164678 Free PMC article. Review.
References
-
- Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR et al. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 2003; 278: 38665–38674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

