Genetic insights into the morass of metastatic heterogeneity
- PMID: 29422598
- PMCID: PMC6290469
- DOI: 10.1038/nrc.2017.126
Genetic insights into the morass of metastatic heterogeneity
Abstract
Tumour heterogeneity poses a substantial problem for the clinical management of cancer. Somatic evolution of the cancer genome results in genetically distinct subclones in the primary tumour with different biological properties and therapeutic sensitivities. The problem of heterogeneity is compounded in metastatic disease owing to the complexity of the metastatic process and the multiple biological hurdles that the tumour cell must overcome to establish a clinically overt metastatic lesion. New advances in sequencing technology and clinical sample acquisition are providing insights into the phylogenetic relationship of metastases and primary tumours at the level of somatic tumour genetics while also illuminating fundamental mechanisms of the metastatic process. In addition to somatically acquired genetic heterogeneity in the tumour cells, inherited population-based genetic heterogeneity can profoundly modify metastatic biology and further complicate the development of effective, broadly applicable antimetastatic therapies. Here, we examine how genetic heterogeneity impacts metastatic disease and the implications of current knowledge for future research endeavours and therapeutic interventions.
Conflict of interest statement
Competing interests
The authors declare no competing financial interests.
Figures
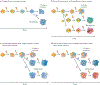


References
-
- Siegel RL, Miller KD & Jemal A Cancer statistics, 2017. CA Cancer J. Clin 67, 7–30 (2017). - PubMed
-
- Spano D, Heck C, De Antonellis P, Christofori G & Zollo M Molecular networks that regulate cancer metastasis. Semin. Cancer Biol 22, 234–249 (2012). - PubMed
-
- Sundquist M, Brudin L & Tejler G Improved survival in metastatic breast cancer 1985–2016. Breast 31, 46–50 (2017). - PubMed
-
- Berry DA et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med 353, 1784–1792 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

