Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity
- PMID: 29422648
- PMCID: PMC5805765
- DOI: 10.1038/s41467-017-02646-2
Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity
Erratum in
-
Publisher Correction: Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity.Nat Commun. 2018 Apr 11;9(1):1498. doi: 10.1038/s41467-018-03769-w. Nat Commun. 2018. PMID: 29643330 Free PMC article.
Abstract
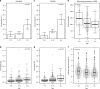
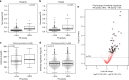
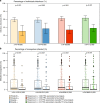
Infection with Plasmodium can elicit antibodies that inhibit parasite survival in the mosquito, when they are ingested in an infectious blood meal. Here, we determine the transmission-reducing activity (TRA) of naturally acquired antibodies from 648 malaria-exposed individuals using lab-based mosquito-feeding assays. Transmission inhibition is significantly associated with antibody responses to Pfs48/45, Pfs230, and to 43 novel gametocyte proteins assessed by protein microarray. In field-based mosquito-feeding assays the likelihood and rate of mosquito infection are significantly lower for individuals reactive to Pfs48/45, Pfs230 or to combinations of the novel TRA-associated proteins. We also show that naturally acquired purified antibodies against key transmission-blocking epitopes of Pfs48/45 and Pfs230 are mechanistically involved in TRA, whereas sera depleted of these antibodies retain high-level, complement-independent TRA. Our analysis demonstrates that host antibody responses to gametocyte proteins are associated with reduced malaria transmission efficiency from humans to mosquitoes.
Conflict of interest statement
P.F. owns stock and is a board member at Antigen Discovery, Inc. The remaining authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

