Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures
- PMID: 29422650
- PMCID: PMC5805762
- DOI: 10.1038/s41598-018-20966-1
Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures
Abstract
A major translational challenge in the fields of therapeutic angiogenesis and regenerative medicine is the need to create functional microvasculature. The purpose of this study was to assess whether a potentially autologous endothelial cell (EC) source derived from human induced pluripotent stem cells (iPSC-ECs) can form the same robust, stable microvasculature as previously documented for other sources of ECs. We utilized a well-established in vitro assay, in which endothelial cell-coated (iPSC-EC or HUVEC) beads were co-embedded with fibroblasts in a 3D fibrin matrix to assess their ability to form stable microvessels. iPSC-ECs exhibited a five-fold reduction in capillary network formation compared to HUVECs. Increasing matrix density reduced sprouting, although this effect was attenuated by distributing the NHLFs throughout the matrix. Inhibition of both MMP- and plasmin-mediated fibrinolysis was required to completely block sprouting of both HUVECs and iPSC-ECs. Further analysis revealed MMP-9 expression and activity were significantly lower in iPSC-EC/NHLF co-cultures than in HUVEC/NHLF co-cultures at later time points, which may account for the observed deficiencies in angiogenic sprouting of the iPSC-ECs. Collectively, these findings suggest fundamental differences in EC phenotypes must be better understood to enable the promise and potential of iPSC-ECs for clinical translation to be realized.
Conflict of interest statement
The authors declare no competing interests.
Figures
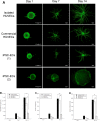

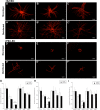
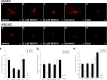
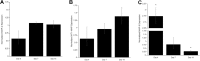

References
-
- Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)- mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. American Journal of Pathology. 1999;154(2):375–84. doi: 10.1016/S0002-9440(10)65284-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

