Effect of Producing Different Phenazines on Bacterial Fitness and Biological Control in Pseudomonas chlororaphis 30-84
- PMID: 29422787
- PMCID: PMC5796749
- DOI: 10.5423/PPJ.FT.12.2017.0277
Effect of Producing Different Phenazines on Bacterial Fitness and Biological Control in Pseudomonas chlororaphis 30-84
Abstract
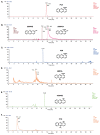
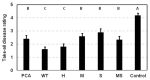
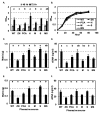
Pseudomonas chlororaphis 30-84 is a biological control agent selected for its ability to suppress diseases caused by fungal pathogens. P. chlororaphis 30-84 produces three phenazines: phenazine-1-carboxylic acid (PCA), 2-hydroxy-phenazine-1-carboxylic acid (2OHPCA) and a small amount of 2-hydroxy-phenazine (2OHPHZ), and these are required for fungal pathogen inhibition and wheat rhizosphere competence. The two, 2-hydroxy derivatives are produced from PCA via the activity of a phenazine-modifying enzyme encoded by phzO. In addition to the seven biosynthetic genes responsible for the production of PCA, many other Pseudomonas strains possess one or more modifying genes, which encode enzymes that act independently or together to convert PCA into other phenazine derivatives. In order to understand the fitness effects of producing different phenazines, we constructed isogenic derivatives of P. chlororaphis 30-84 that differed only in the type of phenazines produced. Altering the type of phenazines produced by P. chlororaphis 30-84 enhanced the spectrum of fungal pathogens inhibited and altered the degree of take-all disease suppression. These strains also differed in their ability to promote extracellular DNA release, which may contribute to the observed differences in the amount of biofilm produced. All derivatives were equally important for survival over repeated plant/harvest cycles, indicating that the type of phenazines produced is less important for persistence in the wheat rhizosphere than whether or not cells produce phenazines. These findings provide a better understanding of the effects of different phenazines on functions important for biological control activity with implications for applications that rely on introduced or native phenazine producing populations.
Keywords: Pseudomonas chlororaphis 30-84; biofilm; biological control; eDNA; phenazine.
Figures

References
-
- Baron SS, Terranova G, Rowe JJ. Molecular mechanism of the antimicrobial action of pyocyanin. Curr Microbiol. 1989;18:223–230. doi: 10.1007/BF01570296. - DOI
LinkOut - more resources
Full Text Sources

