Encouragement-Induced Real-World Upper Limb Use after Stroke by a Tracking and Feedback Device: A Study Protocol for a Multi-Center, Assessor-Blinded, Randomized Controlled Trial
- PMID: 29422881
- PMCID: PMC5788891
- DOI: 10.3389/fneur.2018.00013
Encouragement-Induced Real-World Upper Limb Use after Stroke by a Tracking and Feedback Device: A Study Protocol for a Multi-Center, Assessor-Blinded, Randomized Controlled Trial
Abstract
Introduction: Retraining the paretic upper limb after stroke should be intense and specific to be effective. Hence, the best training is daily life use, which is often limited by motivation and effort. Tracking and feedback technology have the potential to encourage self-administered, context-specific training of upper limb use in the patients' home environment. The aim of this study is to investigate post-intervention and long-term effects of a wrist-worn activity tracking device providing multimodal feedback on daily arm use in hemiparetic subjects beyond 3 months post-stroke.
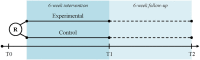
Methods and analysis: A prospective, multi-center, assessor-blinded, Phase 2 randomized controlled trial with a superiority framework. Sixty-two stroke patients will be randomized in two groups with a 1:1 allocation ratio, stratified based on arm paresis severity (Fugl-Meyer Assessment-Upper Extremity subscale <32 and ≥32). The experimental group receives a wrist-worn activity tracking device providing multimodal feedback on daily arm use for 6 weeks. Controls wear an identical device providing no feedback. Sample size: 31 participants per group, based on a difference of 0.75±1.00 points on the Motor Activity Log-14 Item Version, Amount of Use subscale (MAL-14 AOU), 80% power, two-sided alpha of 0.05, and a 10% attrition rate. Outcomes: primary outcome is the change in patient-reported amount of daily life upper limb use (MAL-14 AOU) from baseline to post-intervention. Secondary outcomes are change in upper limb motor function, upper limb capacity, global disability, patient-reported quality of daily life upper limb use, and quality of life from baseline to post-intervention and 6-week follow-up, as well as compliance, activity counts, and safety.
Discussion: The results of this study will show the possible efficacy of a wrist-worn tracking and feedback device on patient-reported amount of daily life upper limb use.
Ethics and dissemination: The study is approved by the Cantonal Ethics Committees Zurich, and Northwest and Central Switzerland (BASEC-number 2017-00948) and registered in https://clinicaltrials.gov (NCT03294187) before recruitment started. This study will be carried out in compliance with the Declaration of Helsinki, ICH-GCP, ISO 14155:2011, and Swiss legal and regulatory requirements. Dissemination will include submission to a peer-reviewed journal, patient and healthcare professional magazines, and congress presentations.
Keywords: daily life; feedback; movement sensor; rehabilitation; stroke; upper limb.
Figures


References
-
- Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYS) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet (2015) 386:2145–91.10.1016/s0140-6736(15)61340-x - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

