The Complement System in Dialysis: A Forgotten Story?
- PMID: 29422906
- PMCID: PMC5788899
- DOI: 10.3389/fimmu.2018.00071
The Complement System in Dialysis: A Forgotten Story?
Abstract
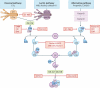
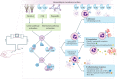
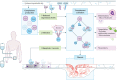
Significant advances have lead to a greater understanding of the role of the complement system within nephrology. The success of the first clinically approved complement inhibitor has created renewed appreciation of complement-targeting therapeutics. Several clinical trials are currently underway to evaluate the therapeutic potential of complement inhibition in renal diseases and kidney transplantation. Although, complement has been known to be activated during dialysis for over four decades, this area of research has been neglected in recent years. Despite significant progress in biocompatibility of hemodialysis (HD) membranes and peritoneal dialysis (PD) fluids, complement activation remains an undesired effect and relevant issue. Short-term effects of complement activation include promoting inflammation and coagulation. In addition, long-term complications of dialysis, such as infection, fibrosis and cardiovascular events, are linked to the complement system. These results suggest that interventions targeting the complement system in dialysis could improve biocompatibility, dialysis efficacy, and long-term outcome. Combined with the clinical availability to safely target complement in patients, the question is not if we should inhibit complement in dialysis, but when and how. The purpose of this review is to summarize previous findings and provide a comprehensive overview of the role of the complement system in both HD and PD.
Keywords: complement; dialysis; hemodialysis; kidney; peritoneal dialysis.
Figures
References
-
- Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet (2016) 388:294–306. 10.1016/S0140-6736(16)30448-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

