Direct conversion of human fibroblasts into functional Leydig-like cells by SF-1, GATA4 and NGFI-B
- PMID: 29423003
- PMCID: PMC5801356
Direct conversion of human fibroblasts into functional Leydig-like cells by SF-1, GATA4 and NGFI-B
Abstract
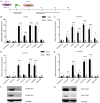
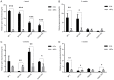
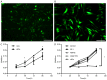
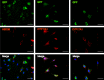
The reprogramming of fibroblasts to induced pluripotent stem cells raises the possibility that a somatic cell can be reprogrammed to an alternative, differentiated fate without first becoming a stem/progenitor cell. Recent work has shown that fibroblasts can be reprogrammed to other, terminally differentiated cells with a combination of several transcription factors. Here, we report that a combination of four developmental transcription factors; GATA4, SF-1, NGFI-B, and COUP TF2; efficiently reprogrammed human foreskin fibroblasts into functional induced Leydig-like cells (iLCs). The iLCs expressed Leydig-specific markers and secreted testosterone in vitro. We found that GATA4 and SF-1 were particularly critical for Leydig-specific markers expression and that GATA4, SF-1, and NGFI-B were necessary to generate functional iLCs that secreted testosterone. These findings demonstrate that fibroblasts can be directly converted into iLCs with a few, defined factors and may provide insight into potential therapies to treat testosterone deficiency.
Keywords: Human foreskin fibroblasts; induced Leydig-like cells; reprogramming.
Conflict of interest statement
None.
Figures

Similar articles
-
Generation of Leydig-like cells: approaches, characterization, and challenges.Asian J Androl. 2022 Jul-Aug;24(4):335-344. doi: 10.4103/aja202193. Asian J Androl. 2022. PMID: 35017389 Free PMC article. Review.
-
CRISPR/dCas9-mediated activation of multiple endogenous target genes directly converts human foreskin fibroblasts into Leydig-like cells.J Cell Mol Med. 2019 Sep;23(9):6072-6084. doi: 10.1111/jcmm.14470. Epub 2019 Jul 2. J Cell Mol Med. 2019. PMID: 31264792 Free PMC article.
-
Leydig-like cells derived from reprogrammed human foreskin fibroblasts by CRISPR/dCas9 increase the level of serum testosterone in castrated male rats.J Cell Mol Med. 2020 Apr;24(7):3971-3981. doi: 10.1111/jcmm.15018. Epub 2020 Mar 11. J Cell Mol Med. 2020. PMID: 32160419 Free PMC article.
-
Direct Reprogramming of Mouse Fibroblasts toward Leydig-like Cells by Defined Factors.Stem Cell Reports. 2017 Jan 10;8(1):39-53. doi: 10.1016/j.stemcr.2016.11.010. Epub 2016 Dec 22. Stem Cell Reports. 2017. PMID: 28017657 Free PMC article.
-
Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications.Circ Res. 2015 Apr 10;116(8):1378-91. doi: 10.1161/CIRCRESAHA.116.305374. Circ Res. 2015. PMID: 25858064 Review.
Cited by
-
Small Molecule Cocktails Promote Fibroblast-to-Leydig-like Cell Conversion for Hypogonadism Therapy.Pharmaceutics. 2023 Oct 13;15(10):2456. doi: 10.3390/pharmaceutics15102456. Pharmaceutics. 2023. PMID: 37896216 Free PMC article.
-
Adaptation of Human Testicular Niche Cells for Pluripotent Stem Cell and Testis Development Research.Tissue Eng Regen Med. 2020 Apr;17(2):223-235. doi: 10.1007/s13770-020-00240-0. Epub 2020 Feb 29. Tissue Eng Regen Med. 2020. PMID: 32114677 Free PMC article.
-
Generation of Leydig-like cells: approaches, characterization, and challenges.Asian J Androl. 2022 Jul-Aug;24(4):335-344. doi: 10.4103/aja202193. Asian J Androl. 2022. PMID: 35017389 Free PMC article. Review.
-
Rebuilding Tendons: A Concise Review on the Potential of Dermal Fibroblasts.Cells. 2020 Sep 8;9(9):2047. doi: 10.3390/cells9092047. Cells. 2020. PMID: 32911760 Free PMC article. Review.
-
Trps1 acts as a regulator of Sf-1 transcription and testosterone synthesis in mouse Leydig cells.Cell Biol Toxicol. 2023 Dec;39(6):3141-3157. doi: 10.1007/s10565-023-09823-8. Epub 2023 Aug 2. Cell Biol Toxicol. 2023. PMID: 37531013
References
-
- Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299:23–31. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
