Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis
- PMID: 29423006
- PMCID: PMC5801359
Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis
Abstract
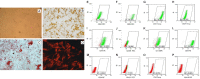
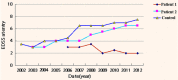
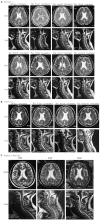
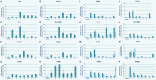
To investigate the clinical efficacy and safety of umbilical cord mesenchymal stem cell (UCMSC) transplantation for treating multiple sclerosis (MS), the patients with MS were recruited and treated with UCMSC. The procedure of preparing UCMSC was in accordance with the standards formulated by the International Society for Cell Biology. Cell surface markers, multiple differentiation potential and safety of UCMSC for transplantation were detected. The number of cells in each infusion was 1 to 2×106 cells/kg. Patients were recruited in accordance with the standards of the International Mesenchymal Stem Cells Transplantation Study Group. After treatment, the clinical therapeutic effects including symptoms, vital signs, clinical attacks, magnetic resonance imaging (MRI), neurological function scores and adverse reactions such as fever, dizziness, and vascular irritation were monitored and evaluated. In addition, the regulatory effects of UCMSC on immune system of patients were also assessed. The results showed that the patients' symptoms were improved after UCMSC transplantation. No clinical attacks occurred during transplantation. MRI revealed a reduced number of foci and Expanded Disability Status Scale scores were decreased. Some of patients had adverse reactions after transplantation. These adverse effects were not serious and lasted short duration, thus no intervention was conducted and let it be eliminated by itself. The mRNA expression of CD86, IL-2, CTLA-4, and HLADRB1 in peripheral blood was significantly decreased after UCMSC transplantation (P < 0.05). Based on our present studies, UCMSCs would be considered as a safe and alternative option for treatment of MS.
Keywords: Umbilical cord mesenchymal stem cell; clinical efficacy; multiple sclerosis; transplantation.
Conflict of interest statement
None.
Figures

Similar articles
-
Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury.Brain Res. 2016 Jul 1;1642:426-435. doi: 10.1016/j.brainres.2016.04.025. Epub 2016 Apr 13. Brain Res. 2016. PMID: 27085204
-
Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis.J Transl Med. 2018 Mar 9;16(1):57. doi: 10.1186/s12967-018-1433-7. J Transl Med. 2018. PMID: 29523171 Free PMC article. Clinical Trial.
-
Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells.Transplantation. 2011 Jun 27;91(12):1412-6. doi: 10.1097/TP.0b013e31821aba18. Transplantation. 2011. PMID: 21494176
-
Human Umbilical Cord-Derived Stem Cells: Isolation, Characterization, Differentiation, and Application in Treating Diabetes.Crit Rev Biomed Eng. 2018;46(5):399-412. doi: 10.1615/CritRevBiomedEng.2018027377. Crit Rev Biomed Eng. 2018. PMID: 30806260 Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Stem cell-based therapy for human diseases.Signal Transduct Target Ther. 2022 Aug 6;7(1):272. doi: 10.1038/s41392-022-01134-4. Signal Transduct Target Ther. 2022. PMID: 35933430 Free PMC article. Review.
-
Mesenchymal stromal cells attenuate multiple sclerosis via IDO-dependent increasing the suppressive proportion of CD5+ IL-10+ B cells.Am J Transl Res. 2019 Sep 15;11(9):5673-5688. eCollection 2019. Am J Transl Res. 2019. PMID: 31632539 Free PMC article.
-
Menstrual blood-derived mesenchymal stromal cells efficiently ameliorate experimental autoimmune encephalomyelitis by inhibiting T cell activation in mice.Stem Cell Res Ther. 2022 Apr 11;13(1):155. doi: 10.1186/s13287-022-02838-8. Stem Cell Res Ther. 2022. PMID: 35410627 Free PMC article.
-
Safety and Clinical Efficacy of Mesenchymal Stem Cell Treatment in Traumatic Spinal Cord Injury, Multiple Sclerosis and Ischemic Stroke - A Systematic Review and Meta-Analysis.Front Neurol. 2022 May 30;13:891514. doi: 10.3389/fneur.2022.891514. eCollection 2022. Front Neurol. 2022. PMID: 35711260 Free PMC article.
-
Tissue engineering strategies combining molecular targets against inflammation and fibrosis, and umbilical cord blood stem cells to improve hampered muscle and skin regeneration following cleft repair.Med Res Rev. 2020 Jan;40(1):9-26. doi: 10.1002/med.21594. Epub 2019 May 18. Med Res Rev. 2020. PMID: 31104334 Free PMC article. Review.
References
-
- Lei XD, Xu F, Li YH. The research progress of tumor necrosis factor alpha in multiple sclerosis and remyelination. Tianjin Yiyao. 2014;11:1141–1143.
-
- Mohajeri M, Farazmand A, Mohyeddin Bonab M, Nikbin B, Minagar A. FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran J Allergy Asthma Immunol. 2011;10:155–161. - PubMed
-
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–394. - PubMed
-
- Yamout B, Hourani R, Salti H, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227:185–189. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
