A novel multiplex detection array revealed systemic complement activation in oral squamous cell carcinoma
- PMID: 29423024
- PMCID: PMC5790441
- DOI: 10.18632/oncotarget.22963
A novel multiplex detection array revealed systemic complement activation in oral squamous cell carcinoma
Abstract
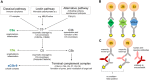
Oral squamous cell carcinoma (OSCC) is one of the most common tumors within the oral cavity. Early diagnosis and prognosis tools are urgently needed. This study aimed to investigate the activation of the complement system in OSCC patients as potential biomarker. Therefore, an innovative complement activation array was developed. Characterized antibodies detecting the complement activation specific epitopes C3a, C5a and sC5b-9 along with control antibodies were implemented into a suspension bead array. Human serum from a healthy (n = 46) and OSCC patient (n = 57) cohort were used to investigate the role of complement activation in oral tumor progression. The novel multiplex assay detected C3a, C5a and sC5b-9 from a minimal sample volume of human tears, aqueous humor and blood samples. Limits of detection were 0.04 ng/mL for C3a, 0.03 ng/mL for C5a and 18.9 ng/mL for sC5b-9, respectively. Biological cut-off levels guaranteed specific detections from serum. The mean serum concentration of a healthy control cohort was 680 ng/mL C3a, 70 ng/mL C5a and 2247 ng/mL sC5b-9, respectively. The assay showed an intra-assay precision of 2.9-6.4% and an inter-assay precision of 9.2-18.2%. Increased systemic C5a (p < 0.0001) and sC5b-9 (p = 0.01) concentrations in OSCC patients were determined using the validated multiplex complement assay. Higher C5a concentrations correlated with tumor differentiation and OSCC extension state. Systemic sC5b-9 determination provided a novel biomarker for infiltrating tumor growth and C3a levels were associated with local tumor spreading. Our study suggests that systemic complement activation levels in OSCC patients may be useful to assess disease progression.
Keywords: C3a; C5a; complement proteins; mulitplex assay; oral squamous cell carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures


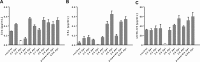


Similar articles
-
Increase of C3a is Associated with Hemorrhagic Propensity in Patients with Immune Thrombocytopenia.Clin Lab. 2017 Apr 1;63(4):765-771. doi: 10.7754/Clin.Lab.2016.161012. Clin Lab. 2017. PMID: 28397467
-
Alterations in plasma complement levels after human ischemic stroke.Neurosurgery. 2006 Jul;59(1):28-33; discussion 28-33. doi: 10.1227/01.NEU.0000219221.14280.65. Neurosurgery. 2006. PMID: 16823297
-
Alterations in systemic complement component 3a and 5a levels in patients with cerebral arteriovenous malformations.J Clin Neurosci. 2011 Sep;18(9):1235-9. doi: 10.1016/j.jocn.2011.02.015. Epub 2011 Jul 13. J Clin Neurosci. 2011. PMID: 21742500
-
Predictive value of complement activation fragments C3a and sC5b-9 for development of severe disease in patients with acute pancreatitis.Scand J Gastroenterol. 2003 Oct;38(10):1078-82. doi: 10.1080/00365520310005965. Scand J Gastroenterol. 2003. PMID: 14621284
-
Complement and macrophage crosstalk during process of angiogenesis in tumor progression.J Biomed Sci. 2015 Jul 22;22(1):58. doi: 10.1186/s12929-015-0151-1. J Biomed Sci. 2015. PMID: 26198107 Free PMC article. Review.
Cited by
-
Prediction of Oral Cancer Biomarkers by Salivary Proteomics Data.Int J Mol Sci. 2024 Oct 16;25(20):11120. doi: 10.3390/ijms252011120. Int J Mol Sci. 2024. PMID: 39456901 Free PMC article.
-
Tumour-cell-derived complement components C1r and C1s promote growth of cutaneous squamous cell carcinoma.Br J Dermatol. 2020 Mar;182(3):658-670. doi: 10.1111/bjd.18095. Epub 2019 Jul 28. Br J Dermatol. 2020. PMID: 31049937 Free PMC article.
-
Role of C5b-9 and RGC-32 in Cancer.Front Immunol. 2019 May 9;10:1054. doi: 10.3389/fimmu.2019.01054. eCollection 2019. Front Immunol. 2019. PMID: 31156630 Free PMC article. Review.
-
Comparative sera proteomics analysis of differentially expressed proteins in oral squamous cell carcinoma.PeerJ. 2021 Jun 10;9:e11548. doi: 10.7717/peerj.11548. eCollection 2021. PeerJ. 2021. PMID: 34178453 Free PMC article.
-
Analyzing Pooled Microarray Gene Expression Data to Uncover Common Pathways in Periodontitis and Oral Squamous Cell Carcinoma from the Gene Expression Omnibus.J Pharm Bioallied Sci. 2024 Apr;16(Suppl 2):S1515-S1521. doi: 10.4103/jpbs.jpbs_1180_23. Epub 2024 Apr 16. J Pharm Bioallied Sci. 2024. PMID: 38882729 Free PMC article.
References
-
- Saba NF, Goodman M, Ward K, Flowers C, Ramalingam S, Owonikoko T, Chen A, Grist W, Wadsworth T, Beitler JJ, Khuri FR, Shin DM. Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue, and tonsils: a surveillance, epidemiology and end results program-based analysis. Oncology. 2011;81:12–20. - PMC - PubMed
-
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65:401–21. - PubMed
-
- Gupta B, Johnson NW, Kumar N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology. 2016;91:13–23. - PubMed
-
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. - PubMed
-
- Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Maso LD, Daudt AW, Fabianova E, Wunsch-Filho V, Franceschi S, Hayes RB, Herrero R, et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. JNCI. 2007;99:777–89. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

