Transforming growth factor-β in stem cells and tissue homeostasis
- PMID: 29423331
- PMCID: PMC5802812
- DOI: 10.1038/s41413-017-0005-4
Transforming growth factor-β in stem cells and tissue homeostasis
Abstract
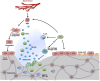
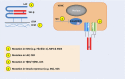
TGF-β 1-3 are unique multi-functional growth factors that are only expressed in mammals, and mainly secreted and stored as a latent complex in the extracellular matrix (ECM). The biological functions of TGF-β in adults can only be delivered after ligand activation, mostly in response to environmental perturbations. Although involved in multiple biological and pathological processes of the human body, the exact roles of TGF-β in maintaining stem cells and tissue homeostasis have not been well-documented until recent advances, which delineate their functions in a given context. Our recent findings, along with data reported by others, have clearly shown that temporal and spatial activation of TGF-β is involved in the recruitment of stem/progenitor cell participation in tissue regeneration/remodeling process, whereas sustained abnormalities in TGF-β ligand activation, regardless of genetic or environmental origin, will inevitably disrupt the normal physiology and lead to pathobiology of major diseases. Modulation of TGF-β signaling with different approaches has proven effective pre-clinically in the treatment of multiple pathologies such as sclerosis/fibrosis, tumor metastasis, osteoarthritis, and immune disorders. Thus, further elucidation of the mechanisms by which TGF-β is activated in different tissues/organs and how targeted cells respond in a context-dependent way can likely be translated with clinical benefits in the management of a broad range of diseases with the involvement of TGF-β.
Figures

References
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF beta activation. J. Cell Sci. 2003;116:217–224. - PubMed
-
- Pfeilschifter J, Bonewald L, Mundy GR. Characterization of the latent transforming growth factor beta complex in bone. J. Bone Miner. Res. 1990;5:49–58. - PubMed
-
- Pedrozo HA, et al. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-beta1 from the extracellular matrix of growth plate chondrocytes. Endocrinology. 1999;140:5806–5816. - PubMed
-
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta. 2008;1782:197–228. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

