Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial
- PMID: 29423520
- PMCID: PMC5885185
- DOI: 10.1001/jamaoncol.2017.5179
Cisplatin Chemoradiotherapy vs Radiotherapy in FIGO Stage IIIB Squamous Cell Carcinoma of the Uterine Cervix: A Randomized Clinical Trial
Abstract
Importance: The evidence for concurrent chemoradiotherapy (CT-RT) in International Federation of Gynecology and Obstetrics (FIGO) stage IIIB squamous cell carcinoma of the uterine cervix is not robust. This study reports the final results of a randomized clinical trial of concurrent cisplatin-based CT-RT and radiotherapy alone (RT) in women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix.
Objective: To investigate the benefit of concurrent CT-RT in FIGO stage IIIB squamous cell carcinoma of the uterine cervix.
Design, setting, and participants: This phase 3 open-label randomized clinical trial accrued 850 women in Mumbai, India, between July 7, 2003, and September 22, 2011. Of 2121 screened, 850 women with FIGO stage IIIB squamous cell carcinoma of the uterine cervix suitable for concurrent cisplatin chemotherapy were randomly assigned to CT-RT and RT using block randomization (1:1). The data were updated for a minimum follow-up period of 5 years until December 2016. The final analyses were performed in February and March 2017. This single-institution study was conducted at a tertiary cancer center setting.
Interventions: Randomization to receive RT (RT arm), comprising a combination of external beam RT (50 Gy in 25 fractions over 5 weeks) and brachytherapy, or to receive in addition to the same RT concurrent weekly cisplatin chemotherapy (40 mg/m2 per week) (CT-RT arm).
Main outcomes and measures: The primary end point was 5-year disease-free survival (DFS), defined as the time between the date of randomization and the date of any recurrence or death (whichever occurred first) in the intent-to-treat population.
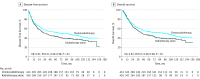
Results: This trial included 424 women assigned to CT-RT (mean [SD] age, 49.4 [7.9] years) and 426 women assigned to RT (mean [SD] age, 49.3 [7.9] years). At a median follow-up of 88 months (interquartile range, 61.3-113.1 months), there were 222 recurrences and 213 deaths in the CT-RT arm and 252 recurrences and 243 deaths in the RT arm. The 5-year DFS was significantly higher in the CT-RT arm (52.3%; 95% CI, 52.2%-52.4%) compared with the RT arm (43.8%; 95% CI, 43.7%-43.9%), with a hazard ratio for relapse or death of 0.81 (95% CI, 0.68-0.98) (P = .03). Similarly, the 5-year overall survival (OS) was significantly higher in the CT-RT arm (54.0%; 95% CI, 53.9%-54.1%) compared with the RT arm (46.0%; 95% CI, 45.9%-46.1%), with a hazard ratio for death of 0.82 (95% CI, 0.68-0.98; P = .04). After adjusting for prognostic factors, CT-RT continued to be significantly superior to RT for DFS and OS. There was a higher incidence of acute hematological adverse effects in the CT-RT arm.
Conclusions and relevance: Chemoradiotherapy using weekly cisplatin results in significantly better DFS and OS compared with RT in women with stage IIIB squamous cell carcinoma of the uterine cervix. This study provides level 1 evidence in the largest clinical trial reported so far in favor of concurrent weekly cisplatin chemotherapy in this setting.
Trial registration: clinicaltrials.gov Identifier: NCT00193791.
Conflict of interest statement
Figures

Comment in
-
Concurrent Chemoradiotherapy for Stage IIIB Cervical Cancer-Global Impact Through Power.JAMA Oncol. 2018 Apr 1;4(4):514-515. doi: 10.1001/jamaoncol.2017.5078. JAMA Oncol. 2018. PMID: 29423514 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr. Accessed May 2017.
-
- National Centre for Disease Informatics and Research (NCDIR) Time Trends in Cancer Incidence Rates 1982-2010. Bangalore: Indian Council of Medical Research; July 2013.
-
- Shrivastava S, Mahantshetty U, Engineer R, Tongaonkar H, Kulkarni J, Dinshaw K. Treatment and outcome in cancer cervix patients treated between 1979 and 1994: a single institutional experience. J Cancer Res Ther. 2013;9(4):672-679. - PubMed
-
- Keys HM, Bundy BN, Stehman FB, et al. . Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161. - PubMed
-
- Rose PG, Bundy BN, Watkins EB, et al. . Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

