Inhaled mannitol for cystic fibrosis
- PMID: 29424930
- PMCID: PMC6491147
- DOI: 10.1002/14651858.CD008649.pub3
Inhaled mannitol for cystic fibrosis
Update in
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2020 May 1;5(5):CD008649. doi: 10.1002/14651858.CD008649.pub4. Cochrane Database Syst Rev. 2020. PMID: 32358807 Free PMC article.
Abstract
Background: Several agents are used to clear secretions from the airways of people with cystic fibrosis. Mannitol increases mucociliary clearance, but its exact mechanism of action is unknown. The dry powder formulation of mannitol may be more convenient and easier to use compared with established agents which require delivery via a nebuliser. Phase III trials of inhaled dry powder mannitol for the treatment of cystic fibrosis have been completed and it is now available in Australia and some countries in Europe. This is an update of a previous review.
Objectives: To assess whether inhaled dry powder mannitol is well tolerated, whether it improves the quality of life and respiratory function in people with cystic fibrosis and which adverse events are associated with the treatment.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic databases, handsearching relevant journals and abstracts from conferences.Date of last search: 28 September 2017.
Selection criteria: All randomised controlled studies comparing mannitol with placebo, active inhaled comparators (for example, hypertonic saline or dornase alfa) or with no treatment.
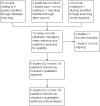
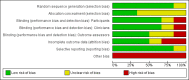
Data collection and analysis: Authors independently assessed studies for inclusion, carried out data extraction and assessed the risk of bias in included studies. The quality of the evidence was assessed using GRADE.
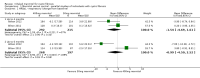
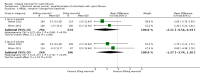
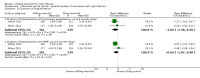
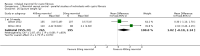
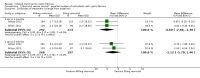
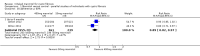
Main results: Six studies (reported in 50 publications) were included with a total of 784 participants.Duration of treatment in the included studies ranged from 12 days to six months, with open-label treatment for an additional six months in two of the studies. Five studies compared mannitol with control (a very low dose of mannitol or non-respirable mannitol) and the final study compared mannitol to dornase alfa alone and to mannitol plus dornase alfa. Two large studies had a similar parallel design and provided data for 600 participants, which could be pooled where data for a particular outcome and time point were available. The remaining studies had much smaller sample sizes (ranging from 22 to 95) and data could not be pooled due to differences in design, interventions and population.Pooled evidence from the two large parallel studies was judged to be of low to moderate quality and from the smaller studies was judged to be of low to very low quality. In all studies, there was an initial test to see if participants tolerated mannitol, with only those who could tolerate the drug being randomised; therefore, the study results are not applicable to the cystic fibrosis population as a whole.While the published papers did not provide all the data required for our analysis, additional unpublished data were provided by the drug's manufacturer and the author of one of the studies.Pooling the large parallel studies comparing mannitol to control, up to and including six months, lung function (forced expiratory volume at one second) measured in both mL and % predicted was significantly improved in the mannitol group compared to the control group (moderate-quality evidence). Beneficial results were observed in these studies in adults and in both concomitant dornase alfa users and non-users in these studies. In the smaller studies, statistically significant improvements in lung function were also observed in the mannitol groups compared to the non-respirable mannitol groups; however, we judged this evidence to be of low to very low quality.For the comparisons of mannitol and control, we found no consistent differences in health-related quality of life in any of the domains except for burden of treatment, which was less for mannitol up to four months in the two pooled studies of a similar design; this difference was not maintained at six months. It should be noted that the tool used to measure health-related quality of life was not designed to assess mucolytics and pooling of the age-appropriate tools (as done in some of the included studies) may not be valid so results were judged to be low to very low quality and should be interpreted with caution. Cough, haemoptysis, bronchospasm, pharyngolaryngeal pain and post-tussive vomiting were the most commonly reported side effects in both treatment groups. Where rates of adverse events could be compared, statistically no significant differences were found between mannitol and control groups; although some of these events may have clinical relevance for people with CF.For the comparisons of mannitol to dornase alfa alone and to mannitol plus dornase alfa, very low-quality evidence from a 12-week cross-over study of 28 participants showed no statistically significant differences in the recorded domains of health-related quality of life or measures of lung function. Cough was the most common side effect in the mannitol alone arm but there was no occurrence of cough in the dornase alfa alone arm and the most commonly reported reason of withdrawal from the mannitol plus dornase alfa arm was pulmonary exacerbations.In terms of secondary outcomes of the review (pulmonary exacerbations, hospitalisations, symptoms, sputum microbiology), evidence provided by the included studies was more limited. For all comparisons, no consistent statistically significant and clinically meaningful differences were observed between mannitol and control treatments (including dornase alfa).
Authors' conclusions: There is moderate-quality evidence to show that treatment with mannitol over a six-month period is associated with an improvement in some measures of lung function in people with cystic fibrosis compared to control. There is low to very low-quality evidence suggesting no difference in quality of life for participants taking mannitol compared to control. This review provides very low-quality evidence suggesting no difference in lung function or quality of life comparing mannitol to dornase alfa alone and to mannitol plus dornase alfa.The clinical implications from this review suggest that mannitol could be considered as a treatment in cystic fibrosis; but further research is required in order to establish who may benefit most and whether this benefit is sustained in the longer term. Furthermore, studies comparing its efficacy against other (established) mucolytic therapies need to be undertaken before it can be considered for mainstream practice.
Conflict of interest statement
Sarah Nevitt has no potential conflict of interest to declare.
Dr Murray was local principal investigator (and thus recruiting participants) for an inhaled mannitol study run by Pharmaxis. Her institution was paid on a per‐participant basis for participants recruited to the study, but she herself did not benefit financially for running the study.
Dr Thornton was pharmacist at one site for a clinical study of inhaled mannitol in children and young people with CF in the UK. Dr Thornton’s main employment is with NICE, Centre for Guidelines. NICE Centre for Health Technology Evaluation, have published an appraisal of mannitol in CF. The Centre for Guidelines have published a guideline for the management of CF which includes mannitol. Dr Thornton is also a member of the GRADE working group.
Tiffany Dwyer declares she has worked on a trial of hypertonic saline in people with cystic fibrosis and another trial in non‐cystic fibrosis bronchiectasis. Hypertonic saline is a mucolytic agent that works similarly to inhaled mannitol.
Figures



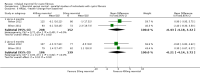
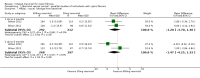
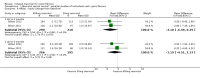
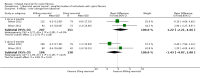



















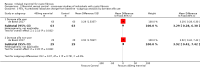

















Update of
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2015 Oct 9;(10):CD008649. doi: 10.1002/14651858.CD008649.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 09;2:CD008649. doi: 10.1002/14651858.CD008649.pub3. PMID: 26451533 Updated.
References
References to studies included in this review
Aitken 2012 {published and unpublished data}
-
- Aitken M, Bilton D, Fox H, Charlton B, CF‐301 &CF‐302SI. Bronchitol (inhaled dry powder mannitol) in adult patients with cystic fibrosis. Journal of Cystic Fibrosis 2012;11 Suppl 1:S68. [Abstract no.: 47; CENTRAL: 962111; CFGD Register: BD198f/BD152h; CRS: 5500125000000024]
-
- Aitken M, Flume PA, Geller DE, Lapey A, Zuckerman J, Boeck K, et al. Phase III study [CF‐302] of inhaled dry powder mannitol (Bronchitol™) in cystic fibrosis ‐ results from the 6 month open label phase. Journal of Cystic Fibrosis 2011;10 Suppl 1:S20. [Abstract no.: 77; CENTRAL: 962109; CFGD Register: BD198b; CRS: 5500125000000467]
-
- Aitken MI, Flume PA, Geller DE, Lapey A, Zuckerman J, Boeck K, et al. Efficacy and safety by age group from the phase III studies of bronchitol (inhaled mannitol) in patients with CF. Pediatric Pulmonology 2011;46 Suppl 34:297. [Abstract no.: 236; CENTRAL: 991565; CFGD Register: BD198j /BD152o; CRS: 5500125000000651]
-
- Aitken ML, Bellon G, Boeck K, Flume PA, Fox HG, Geller DE, et al. Long‐term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. American Journal of Respiratory and Critical Care Medicine 2012;185:645‐52. [CENTRAL: 881656; CFGD Register: BD198c; CRS: 5500100000006189; PUBMED: 22198974] - PubMed
-
- Aitken ML, Flume PA, Geller DE, Lapey A, Zuckerman J, Fox H, et al. Six‐month administration of inhaled mannitol in cystic fibrosis ‐ a safety and efficacy study. Pediatric Pulmonology 2010;45 Suppl 33:299. [Abstract no.: 225; CENTRAL: 962107; CFGD Register: BD198a; CRS: 5500100000006204]
Bilton 2011 {published and unpublished data}
-
- Aitken M, Bilton D, Fox H, Charlton B, CF‐301, CF‐302SI. Bronchitol (inhaled dry powder mannitol) in adult patients with cystic fibrosis. Journal of Cystic Fibrosis 2012;11 Suppl 1:S68. [Abstract no.: 47; CENTRAL: 962111; CFGD Register: BD152h/BD198f; CRS: 5500125000000024]
-
- Aitken MI, Flume PA, Geller DE, Lapey A, Zuckerman J, Boeck K, et al. Efficacy and safety by age group from the phase III studies of bronchitol (inhaled mannitol) in patients with CF. Pediatric Pulmonology 2011;46 Suppl 34:297. [Abstract no.: 236; CENTRAL: 991565; CFGD Register: BD152o/BD198j ; CRS: 5500125000000651]
-
- Bilton D, Aitken M, Boeck K, Fox H, Charlton B, CF‐301, CF‐302 SI. Inhaled dry powder mannitol in cystic fibrosis (CF): impact on pulmonary exacerbations (PEs) in the Phase III studies (CF‐301 & CF‐302). Journal of Cystic Fibrosis 2012;11 Suppl 1:S11. [Abstract no.: WS5.2; CENTRAL: 962110; CFGD Register: BD152i/BD198g; CRS: 5500125000000015]
-
- Bilton D, Aitken M, Flume PA, Geller DE, Lapey A, Zuckerman J, et al. Combined data from the two phase III studies of bronchitol (inhaled dry powder mannitol) in adult cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2011;10 Suppl 1:S20. [Abstract no.: 78; CENTRAL: 962114; CFGD Register: BD152g/BD198e; CRS: 5500125000000468]
-
- Bilton D, Aitken M, Fox H, Charlton B, for the CF301, CF302 SI. Inhaled dry powder mannitol in cystic fibrosis (CF): the microbiology demographics and results from the phase III studies (CF301 and CF302). Journal of Cystic Fibrosis 2011;10 Suppl 1:S20. [Abstract no.: 79; CFGD Register: BD152n/BD198i ; CRS: 5500125000000653]
de Boeck 2017 {published data only}
-
- Crossover trial determining the efficacy of dry powder mannitol to improve lung function in subjects aged 6‐17 years. clinicaltrials.gov/ct2/show/NCT01883531 (accessed 21 April 2015). [Pharmaxis ID: CF204]
-
- Boeck C, Haarman E, Hull J, Lands LC, Moeller A, Munck A, et al. A phase II, randomised, double‐blind, placebo‐controlled study of dry powder mannitol in children with CF (overall and subgroup analyses). Pediatric Pulmonology 2016;51 Suppl 45:273‐4. [Abstract no.: 215; CFGD Register: BD234b]
-
- Boeck K, Haarman E, Hull J, Lands LC, Moeller A, Munck A, et al. A phase II, randomised, double‐blind, placebo‐controlled, crossover study of dry powder mannitol in children with cystic fibrosis (CF). Journal of Cystic Fibrosis 2016;15 Suppl 1:S5. [Abstract no.: WS03.3] - PubMed
-
- Boeck K, Haarman E, Hull J, Lands LC, Moeller A, Munck A, et al. Inhaled dry powder mannitol in children with cystic fibrosis: a randomised efficacy and safety trial. Journal of Cystic Fibrosis 2017;16(3):380‐7. [CFGD Register: BD234c] - PubMed
-
- EUCTR2012‐002699‐14‐GB. Trial of inhaled mannitol in children with cystic fibrosis. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu....
Jaques 2008 {published and unpublished data}
-
- Charlton B, Lassig A. A randomized, blinded, controlled, crossover study of inhaled, dry powder mannitol (bronchitol) in CF. Proceedings of American Thoracic Society Conference; 2006 May 19‐24; California, USA. 2006:A727. [CFGD Register: BD120a]
-
- Charlton B, Lassig A, CF‐201 Study Investigators. Inhaled dry powder mannitol (Bronchitol) improves FEV1 in cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl 1:S11. [CFGD Register: BD120b]
-
- Charlton B, Lassig A, CF‐201 Study Investigators. Inhaled dry powder mannitol (bronchitol) improves FEV1 in cystic fibrosis. Proceedings of TSANZ 2006 Annual Scientific Meeting. 2006:A11. [CFGD Register: BD120c]
-
- Daviskas E, Anderson SD, Jaques A, Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest 2010;137:861‐8. [CFGD Register: BD120e] - PubMed
-
- Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest 2008;133(6):1388‐96. [CFGD Register: BD120d] - PubMed
Middleton 2015 {published data only}
-
- ACTRN12612001167853. A study examining the utility of inhaled mannitol as an additional therapy during an admission to hospital for an acute pulmonary exacerbation in children with cystic fibrosis. http://www.anzctr.org.au/ACTRN12612001167853.aspx.
-
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with CF hospitalised with pulmonary exacerbation. Pediatric Pulmonology 2012;47 Suppl 35:355. [Abstract no.: 368; CENTRAL: 962113; CFGD Register: BD199a; CRS: 5500125000000040]
-
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with cystic fibrosis hospitalised with pulmonary exacerbation. Department of Respiratory Medicine, Children's Hospital at Westmead (www.thoracic.org.au) (accessed 21 Oct 2014) 2014. [CENTRAL: 1012538; CFGD Register: BD199b; CRS: 5500131000000198]
-
- Middleton A, Robinson PD, McKay K, Jaffe A, Selvadurai H. A pilot study of inhaled dry‐powder mannitol during cystic fibrosis‐related pulmonary exacerbation [letter]. European Respiratory Journal 2015;45(2):541‐4. [CENTRAL: 1048444; CFGD Register: BD199c; CRS: 5500050000000185; EMBASE: 2015713766] - PubMed
Minasian 2010 {published and unpublished data}
-
- EUCTR2004‐001888‐21‐GB. A cross‐over comparative study of inhaled mannitol, alone and in combination with daily rhDNase, in children with cystic fibrosis ‐ Inhaled Mannitol in Cystic Fibrosis. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_nu....
-
- Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax 2010;65(1):51‐6. [CFGD Register: BD133b] - PubMed
-
- Minasian CC, Wallis C, Metcalfe C, Bush A. A crossover comparative study of inhaled mannitol, alone and in combination with daily rhDNase, in children with cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl 31:301. [CFGD Register: BD133a]
-
- NCT00117208. Comparison of Inhaled Mannitol and rhDNase in Children With Cystic Fibrosis. https://ClinicalTrials.gov/show/NCT00117208.
References to studies excluded from this review
Robinson 1999 {published data only}
-
- Robinson M, Daviskas E, Eberl S, Baker J, Anderson SD, Bye PT. Effect of inhaling a dry powder of mannitol on mucociliary clearance in adults with cystic fibrosis. Pediatric Pulmonology 1998;26 Suppl 17:280. [CFGD Register: BD84a]
-
- Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. European Respiratory Journal 1999;14(3):678‐85. [CFGD Register: BD84b] - PubMed
-
- Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Pediatric Pulmonology 2000;29(6):484. [CFGD Register: BD84c] - PubMed
Teper 2011 {published data only}
-
- NCT00251056. Mannitol Dose Response Study in Cystic Fibrosis. https://ClinicalTrials.gov/show/NCT00251056.
-
- Teper A, Charlton B. A dose response trial of inhaled mannitol in patients with cystic fibrosis. Canadian Respiratory Journal 2010;17:13B. [CFGD Register: BD200b] - PubMed
-
- Teper A, Jaques A, Charlton B. Inhaled mannitol in patients with cystic fibrosis: a randomised open‐label dose response trial. Journal of Cystic Fibrosis 2011;10:1‐8. [CFGD Register: BD200a] - PubMed
References to ongoing studies
NCT02134353 {published data only}
-
- A safety and efficacy trial of inhaled mannitol in adult cystic fibrosis subjects. clinicaltrials.gov/ct2/show/NCT02134353 (accessed 21 April 2015). [Pharmaxis ID: CF303]
Additional references
Accurso 2007
Anderson 1997
-
- Anderson SD, Brannan J, Spring J, Spalding N, Rodwell LT, Chan K, et al. A new method for bronchial‐provocation testing in asthmatic subjects using a dry powder of mannitol. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):758‐65. - PubMed
Bilton 2012
-
- Bilton D, Aitken ML, Fox H, Charlton B. Predicting sustained response to Bronchitol™ treatment in patients with cystic fibrosis. Pediatric Pulmonology 2012;47 Suppl 35:289‐90. [Abstract no.: A192]
Bilton 2013
-
- Bilton D, Bellon G, Charlton B, Cooper P, Boeck K, Flume PA, et al. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. Journal of Cystic Fibrosis 2013;12(4):367‐76. [CENTRAL: 867634; CFGD Register: BD152d/BD198d; CRS: 5500100000011535; PUBMED: 23234802] - PubMed
Brannan 2003
-
- Brannan JD, Gulliksson M, Anderson SD, Chew N, Kumlin M. Evidence of mast cell activation and leukotriene release after mannitol inhalation. European Respiratory Journal 2003;22(3):491‐6. - PubMed
Button 2013a
-
- Button BM, Finlayson F, Borg B, Ivulich S, Wilson JW. Inhaled mannitol as an adjunct to airway clearance therapy in an adult cystic fibrosis population and clinical lessons learned. Pediatric Pulmonology 2013;48 Suppl 36:356‐7.
Button 2013b
-
- Button BM, Finlayson F, Borg B, Ivulich S, Wilson JW. Clinical impact of inhaled mannitol in an adult cystic fibrosis population. Journal of Cystic Fibrosis 2013;12 Suppl 1:S98. [Abstract no.:: 194]
Bye 2007
-
- Bye PTP, Elkins MR. Other mucoactive agents for cystic fibrosis. Paediatric Respiratory Reviews 2007;8(1):30‐9. - PubMed
CCFF 2002
-
- Canadian Cystic Fibrosis Foundation. Report of the Canadian Patient Data Registry. Canadian Patient Data Registry. Toronto, Ontario, 2002.
CFF 2000
-
- Cystic Fibrosis Foundation. National Patient Registry Annual Data Report 2000. Cystic Fibrosis Foundation, Bethesda, Maryland 2000.
CFF 2005
-
- Cystic Fibrosis Foundation. National Patient Registry Annual Data Report 2005. Cystic Fibrosis Foundation, Bethesda, Maryland 2005.
Daviskas 1997
-
- Daviskas E, Anderson SD, Brannan JD, Chan H‐K, Eberl S, Bautovich G. Inhalation of dry powder mannitol increases mucociliary clearance. European Respiratory Journal 1997;10(11):2449‐54. - PubMed
Daviskas 2001
-
- Daviskas E, Anderson SD, Eberl S, Chan H‐K, Young IH. The 24‐h effect of mannitol on the clearance of mucus in patients with bronchiectasis. Chest 2001;119(2):414‐21. - PubMed
Daviskas 2002
-
- Daviskas E, Anderson SD, Eberl S, Chan H‐K, Young IH, Seale JP. Effects of terbutaline in combination with mannitol on mucociliary clearance. European Respiratory Journal 2002;20(6):1423‐9. - PubMed
Daviskas 2005
-
- Daviskas E, Anderson SD, Gomes K, Briffa P, Cochrane B, Chan H‐K, et al. Inhaled mannitol for the treatment of mucociliary dysfunction in patients with bronchiectasis: effect on lung function, health status and sputum. Respirology 2005;10(1):46‐56. - PubMed
Daviskas 2010
-
- Daviskas E, Anderson SD, Jaques A, Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest 2010;137(4):861‐8. - PubMed
Deeks 2011
-
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dinwiddie 2000
-
- Dinwiddie R. Pathogenesis of lung disease in cystic fibrosis. Respiration 2000;67(1):3‐8. - PubMed
Dodge 2007
-
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK 1947‐2003. European Respiratory Journal 2007;29(3):522‐26. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
EMA 2011
-
- European Medicines Agency. Bronchitol. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001... (accessed 18 June 2015).
Flume 2015
Fuchs 1994
-
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine 1994;331(10):637‐42. - PubMed
Gee 2000
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodson 2000
-
- Hodson ME, Geddes DM. Cystic Fibrosis. Second Edition. Arnold Press, 2000.
Jones 2005
-
- Jones AP, Riley R, Williamson PR, Whitehead A. Meta‐analysis of longitudinal data. Royal Statistical Society Annual Conference. 2005.
McAuley 2000
-
- McAuley DF, Elborn JS. Cystic fibrosis: basic science. Paediatric Respiratory Reviews 2000;1(2):93‐100. - PubMed
NICE 2012
-
- National Institute for Health and Clinical Excellence. Mannitol dry powder for inhalation for treating cystic fibrosis (TA266). National Institute for Health and Clinical Excellence 2012.
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. - PMC - PubMed
Quittner 2015 [pers comm]
-
- Quittner A (Department of Psychology, Miami College of Arts and Sciences, USA). [personal communication]. Conversation with: T Dwyer (Sydney Medical School, Australia) February 2015.
Ratjen 2003
-
- Ratjen J, Doring G. Cystic Fibrosis. Lancet 2003;361:681‐689. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sawicki 2009
SMC 2013
-
- Scottish Medicines Consortium. Mannitol, 400mg, inhalation powder, hard capsule (Bronchitol®) SMC No. (837/13). www.scottishmedicines.org.uk/files/advice/inhaled_mannitol_Bronchitol_FI... (accessed 18 June 2015).
Sterne 2011
-
- Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook forSystematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Suri 2002
Tam 2013
Wanner 1983
-
- Wanner A, Maurer D, Abraham WM, Szepfalusi Z, Sielczak M. Effects of chemical mediators of anaphylaxis on ciliary function. Journal of Clinical Immunology 1983;72(6):663‐7. - PubMed
Wark 2009
Wills 2006
Yang 2016
Zorginstituut 2014
-
- Zorginstituut Nederland. Mannitol (Bronchitol®) for the indication 'adult patients with cystic fibrosis'. Pharmacotherapeutic report, summary. www.zorginstituutnederland.nl/binaries/content/documents/zinl‐www/docume... (accessed 18 June 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

