IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration
- PMID: 29425074
- PMCID: PMC6037454
- DOI: 10.1080/21645515.2018.1438791
IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration
Abstract
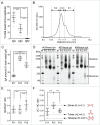
Unlike the current injectable influenza vaccines, intranasally administered influenza vaccines induce influenza virus-specific IgA antibodies in the local respiratory mucosa as well as IgG antibodies in the systemic circulation. Our previous study showed that after five volunteers underwent intranasal administration with inactivated H3N2 or H5N1 vaccines, their IgA antibodies on the upper respiratory tract were present as monomers, dimers, and multimers (trimers and tetramers). Moreover, the multimers associated with the highest virus neutralizing activity. However, it has remained elusive whether a more practical intranasal vaccination strategy could induce the high-performance IgA multimers in the nasal mucosa. In the present study, volunteers were administered with two doses of the intranasal trivalent whole-virus inactivated influenza vaccine and showed that in nasal wash samples the amount of multimeric IgA correlated positively with virus neutralizing titers, indicating that the multimeric IgA antibodies play an important role in the antiviral activity at the nasal mucosa. Surface plasmon resonance analysis of the binding dynamics of nasal wash derived IgA monomers, dimers, and multimers against recombinant trimeric influenza virus HA showed that sample fractions containing IgA multimers dissociated from HA less well than sample fractions without IgA multimers. Thus, IgA multimers may "stick" to the antigen more tightly than the other structures. In summary, intranasal administration of two doses of multivalent inactivated influenza vaccines induced multimeric IgA. Multimerization of mucosal IgA antibodies conferred higher neutralizing activity against viruses in the nasal mucosa, possibly by increasing their cohesion to virus antigens.
Keywords: Secretory IgA antibody; influenza virus; intranasal inactivated influenza vaccine; multimeric SIgA antibody; upper respiratory mucosa.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
